A structure-based modelling approach identifies effective drug combinations for RAS-mutant acute myeloid leukemia
A structure-based modelling approach identifies effective drug combinations for RAS-mutant acute myeloid leukemia
Jones, L.; Rukhlenko, O. S.; Dias, T.; Imoto, H.; Carmody, C.; Wynne, K.; Kholodenko, B. N.; Bond, J.
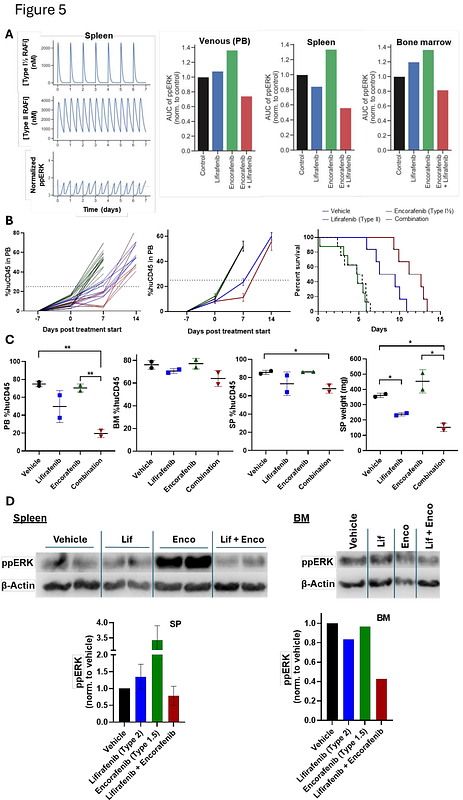
AbstractMutations activating RAS/RAF/MEK/ERK signaling are associated with poor outcome in acute myeloid leukemia (AML), but therapeutic targeting of this pathway is challenging. Here, we employ a structure-based, dynamic RAS pathway model to successfully predict RAF inhibitor (RAFi) combinations which synergistically suppress ERK signaling in RAS-mutant AML. Our in silico models predicted therapeutic synergy of two iterations of conformation-specific RAF inhibitors: Type I[1/2] + Type II and Type I + Type II. Predictions were validated in vitro in AML cell lines and patient samples, with synergy verified by the Loewe Additivity model. Lifirafenib (Type II) + encorafenib (Type I[1/2]) was highly synergistic against both NRAS- and KRAS-mutant lines, while synergy of lifirafenib + SB590885 (Type I) was specific to NRAS-mutants. Immunoblotting confirmed that combination efficacy correlated strongly with decreased RAS pathway activation. Leveraging the pharmacokinetic predictions of our in silico model, both combinations were then assessed in a pre-clinical NRAS-mutant AML patient-derived xenograft (PDX) model, showing significantly improved leukaemia growth delay and event-free survival compared with single agent approaches. Assessment of leukemia burden in bone marrow and spleen during treatment further showed site-specific efficacy against circulating and spleen-resident blasts for both combinations. In summary, we report that our structure based-modelling approach can effectively identify novel, non-obvious, and well-tolerated RAFi combinations that are highly effective against in vitro and in vivo models, thereby suggesting alternative potential therapeutic strategies for high-risk RAS-mutant AML.