Integrated single cell spatial multi-omics landscape of WHO grades 2-4 diffuse gliomas identifies locoregional metabolomic regulators of glioma growth
Integrated single cell spatial multi-omics landscape of WHO grades 2-4 diffuse gliomas identifies locoregional metabolomic regulators of glioma growth
Ma, Y.; Ayadhury, S.; Singh, S.; Vashishath, Y.; Ozdemir, C.; McKee, T.; Nguyen, N.; Basi, A.; Mak, D.; Gomez, J.; Huse, J.; Noor, S.; Winkowski, D.; Baird, R.; Weinberg, J.; Lang, F.; Burks, J.; Bozdag, S.; Seeley, E.; Ene, C.
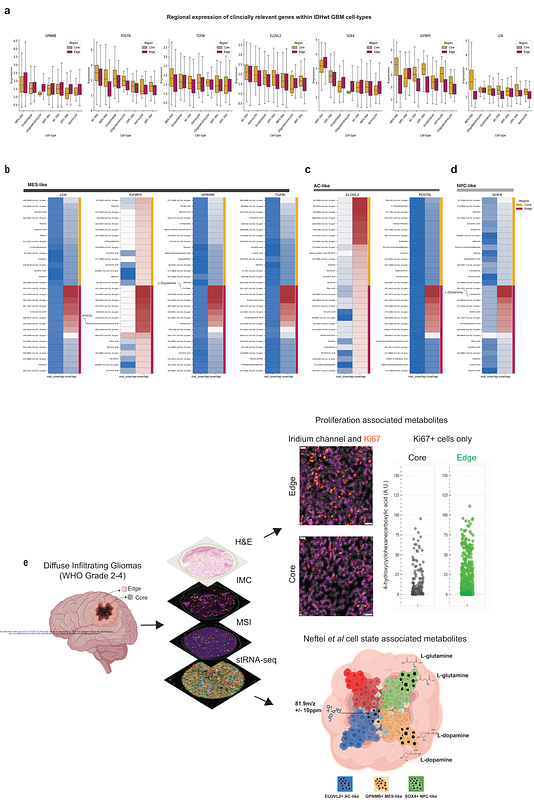
AbstractDiffuse infiltrating gliomas are aggressive tumors of the central nervous system driven by intra-tumoral heterogeneity and aberrant normal-tumor cell-cell interactions. Grade specific and locoregional metabolic dependencies driving aberrant cell-states linked to treatment resistance, seizures and infiltration of gliomas remain elusive. Here, we applied spatial transcriptomics (stRNAseq), imaging mass cytometry (IMC) and mass spectrometry imaging (MSI; metabolites, peptides and glycans) to the core and edge tumor tissue from patients with World Health Organization (WHO) grades 2-4 diffuse infiltrating gliomas including isocitrate dehydrogenase (IDH) mutant oligodendrogliomas (WHO Grades 2 and 3) and IDH wildtype astrocytomas including anaplastic astrocytoma (prior 2016 WHO histological grade 3) and glioblastoma (GBM, WHO grade 4) stRNAseq identified regions-specific differentially expressed genes with significant overall survival implications particularly in IDH wildtype GBM. Integration of stRNA seq and MSI-derived metabolite expression demonstrated enrichment of L-glutamine in SOX4+ Neural progenitor-like (NPC-like) cells and DL-dopamine in GPNMB+ Mesenchymal-like (MES-like) GBM cells at the tumor edge relative to the core. Our results uncover clinically relevant and locoregional cell state-specific metabolites that may contribute to GBM proliferation, infiltration and seizures. This comprehensive pan-diffuse infiltrating glioma multi-omics study could serve as a resource for uncovering region-specific metabolic vulnerabilities encompassing metabolites, glycans and peptides within clinically transcriptionally defined cell states across WHO 2-4 diffuse glioma.