Exposure to per- and polyfluoroalkyl substances elicits cell type-specific impacts on p53 and TGF-β signaling pathways
Exposure to per- and polyfluoroalkyl substances elicits cell type-specific impacts on p53 and TGF-β signaling pathways
Ding, H.; Slack, M.; McClure, H.; Gu, W.; Nabinger, C.; Koviazina, R.; Gu, H.; Kappes, F.; Schultz, T.; Somarelli, J.; Tsigkou, A.
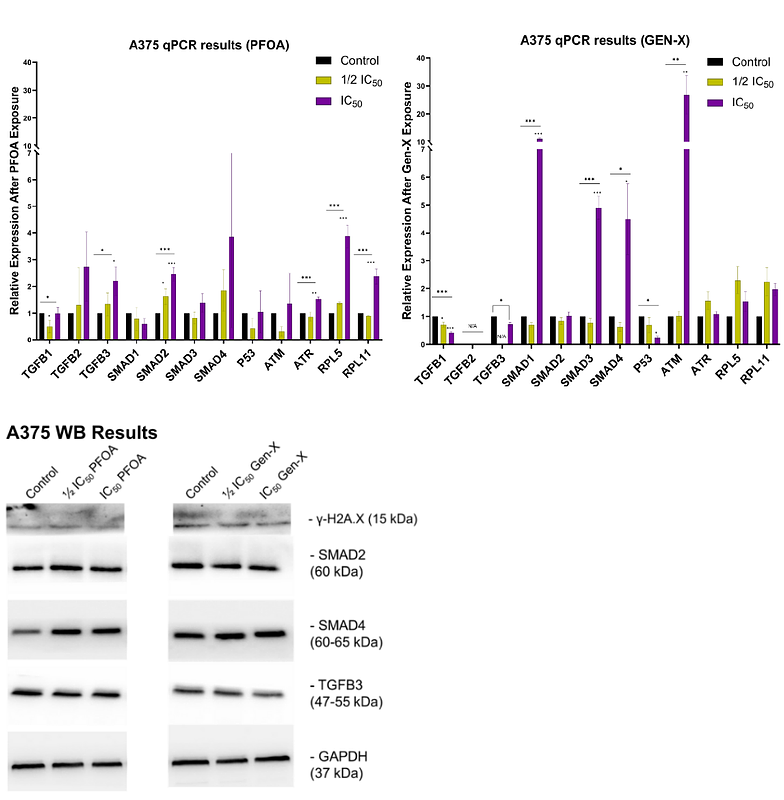
AbstractPer- and polyfluoroalkyl substances (PFAS) are a class of synthetic chemicals extensively used as plastic additives. Their environmental persistence and potential for bioaccumulation have raised significant toxicological concerns. This study evaluates the cytotoxic and molecular effects of Perfluorooctanoic acid (PFOA) and its replacement compound, Perfluoro(2-methyl-3-oxohexanoic) acid (Gen-X), in human-derived skin (A375), liver (HepG2), kidney (SN12C), and colon (SW620) cell lines. We assessed cell viability, gene expression, and perturbations in key cellular stress pathways, with a particular focus on TGF-{beta}/SMAD-mediated inflammation and the p53-driven DNA damage response. Our results demonstrate compound- and cell-type-specific toxicity, with Gen-X displaying reduced cytotoxicity compared to PFOA across all cell types. Molecular analyses revealed that both PFAS compounds induced alterations in the TGF-{beta}/SMAD pathway, consistent with a pro-inflammatory cellular state. Additionally, we observed activation of the DNA damage response, as evidenced by increased expression of {gamma}-H2A.X, ATM, ATR, and p53, alongside ribosomal stress-related changes in RPL5 and RPL11. Notably, while skin and liver cells exhibited similar response profiles, kidney and colon cells showed divergent modulation of SMAD signaling, suggesting tissue-specific susceptibility and mechanistic differences. These findings contribute to a deeper understanding of the differential toxicological profiles of legacy and replacement PFAS, with implications for health risk assessment and regulatory policy.