Netrin-1 regulates colorectal cancer stem cell self-renewal via a TFF3 dependent paracrine survival mechanism
Netrin-1 regulates colorectal cancer stem cell self-renewal via a TFF3 dependent paracrine survival mechanism
Brisset, M.; Radkova, K.; Paradisi, A.; Stephan, L.; Wagner, R.; Degletagne, C.; Luiggi, F.; Frydman, L.; Heriot, A.; Behrenbruch, C.; Vu, T.; Mehlen, P.; Hollande, F.
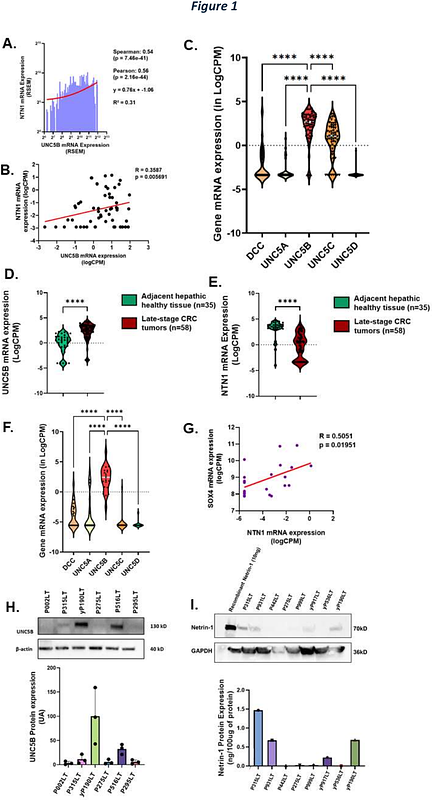
AbstractBackground: Metastatic colorectal cancer (mCRC) is associated with high recurrence rates and resistance to conventional treatments, largely driven by cancer stem cells (CSCs) that contribute to tumor progression and therapeutic evasion. This study aims to investigate the role of netrin-1 and its dependence receptor UNC5B in regulating CSC self-renewal in mCRC and explore their potential as therapeutic targets. Methods: We used patient-derived liver metastasis organoids (PDOs) to examine the effects of netrin-1 on CSC self-renewal. The role of UNC5B was evaluated by silencing its expression using CRISPR and assessing the impact on CSC apoptosis in response to an anti-netrin-1 blocking antibody (NP137) using extreme limiting dilution assays (ELDAs). Single-cell RNA sequencing was employed to explore the molecular mechanisms behind netrin-1/UNC5B regulation of CSC fate. Clinical data from a patient with mCRC were used to validate the findings. Results: Netrin-1 promoted CSC self-renewal by inhibiting apoptosis, a process reversed by NP137. UNC5B was identified as the primary receptor mediating this effect, as its silencing eliminated Netrin-1- induced self-renewal. Trefoil Factor 3 (TFF3), secreted by UNC5B-expressing cells, plays a key role in netrin-1-induced CSC self-renewal. Clinical trial data from a patient with mCRC showed a reduction in TFF3 and stemness genes expression after treatment with NP137. Furthermore, combining NP137 with FOLFOX chemotherapy enhanced cell death and inhibited tumor growth in PDO xenograft models. Conclusion: This study identifies the netrin-1/UNC5B/TFF3 axis as a critical regulator of CSC self-renewal in mCRC and suggests that targeting this pathway with NP137, in combination with chemotherapy, could provide a promising therapeutic approach for mCRC patients.