Context-Dependent Synergism and Antagonism between IGF2BP1/3 and METTL3/14 in m6A-mediated mRNA regulation
Context-Dependent Synergism and Antagonism between IGF2BP1/3 and METTL3/14 in m6A-mediated mRNA regulation
Bhardwaj, R.; Bhakhri, H.; Palanichamy, J. K.
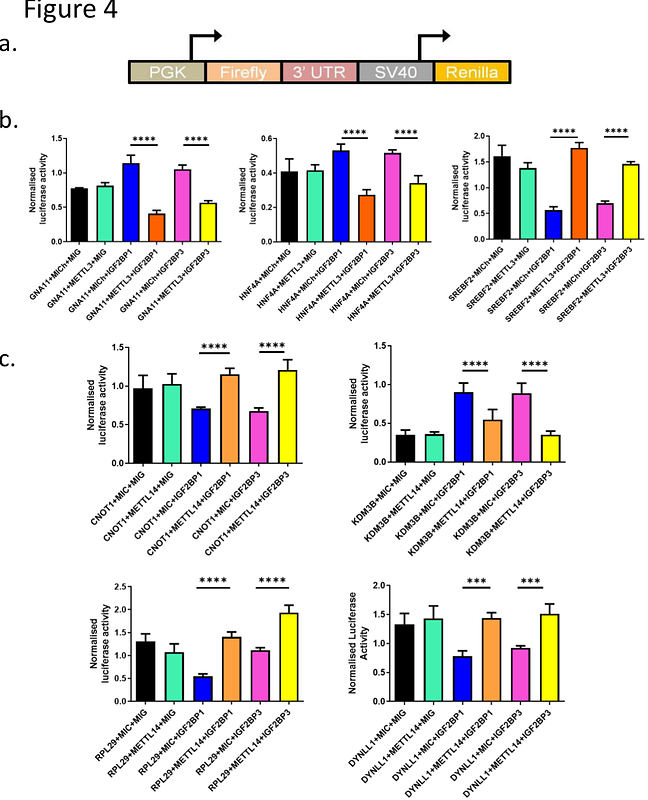
AbstractN-6 Methyl Adenosine (m6A) methylation is primarily found in the 3\'-UTRs of mRNAs, near the stop codon, and at consensus sequence RRACH. The methylation reaction is catalyzed by RNA methyltransferases METTL3 and METTL14, known as writers. Readers recognize the modified mRNA, which influences mRNA stability and translation. Insulin-like growth factor-2 binding proteins 1 and 3 (IGF2BP1 and IGF2BP3) are known m6A readers, promoting mRNA stabilization. Erasers like ALKBH5 and FTO remove m6A modifications. Dysregulation of m6A machinery has been implicated in cancer progression. We analyzed the expression patterns of writers, erasers, and readers (WERs) in multiple public datasets, including NCBI-GEO, TCGA, TARGET, and normal tissue expression data from GTEx. Our findings revealed widespread dysregulation of WERs across various cancers. To investigate whether IGF2BP1/3 and METTL3/14 function synergistically in mRNA stabilization, we identified direct mRNA targets using intersection analyses of IGF2BP1/3 eCLIP and METTL3/14 knockout (KO) datasets on Galaxy server. This analysis identified METTL14-dependent targets (KDM3B, DYNLL1, CNOT1, RPL29) and METTL3-dependent targets (SREBF2, HNF4A, GNA11), all bound by IGF2BP1/3. To validate these interactions, we cloned 3\'-UTRs of these targets downstream of luciferase reporter and assessed mRNA stability following IGF2BP1/3 and METTL3/14 overexpression. Luciferase activity increased for RPL29, DYNLL1, SREBF2, and CNOT1 upon co-expression, indicating IGF2BP1/3-mediated mRNA stabilization in an m6A-dependent manner. In contrast, KDM3B, HNF4A, and GNA11 exhibited reduced luciferase activity, suggesting destabilization. Our study provides novel evidence that m6A readers and writers exhibit synergistic and antagonistic interactions in a target-dependent manner, underscoring the complexity of m6A-mediated gene regulation in oncogenesis.