Proteome-level robustness and the role of a histone-like protein during acute heat shock in the hyperthermophilic archaeon Pyrococcus furiosus
Proteome-level robustness and the role of a histone-like protein during acute heat shock in the hyperthermophilic archaeon Pyrococcus furiosus
Okabe, H.; Miura, M. C.; Sato, A.; Adachi, S.; Kanai, A.
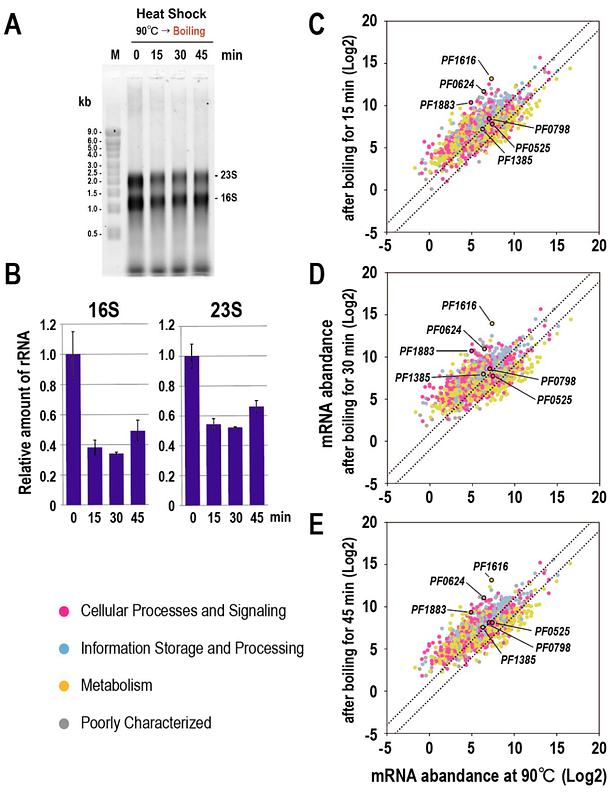
AbstractThe hyperthermophilic archaeon Pyrococcus furiosus thrives in extreme temperatures and exhibits a complex response to heat shock. However, the regulatory dynamics of genetic information during heat shock remain poorly understood. In this study, we exposed P. furiosus (cultured at 90oC) to acute heat shock by boiling (101-102oC) and analyzed its transcriptomic and proteomic responses. The levels of 16S and 23S rRNAs and of total tRNA were decreased by approximately 50%, and pre-tRNA splicing was inhibited, indicating suppression of translation. By contrast, approximately 90% of the proteome remained stable, underscoring the robustness of existing proteins. However, the transcriptome exhibited widespread alterations with limited correlation to the proteome (correlation coefficient r = 0.32), except for a few key proteins. These proteins included PF1883 (small heat shock protein), PF1385 (uracil-DNA glycosylase), and PF1616 (inositol-1-phosphate synthase), which are involved in protein chaperoning, stress-related metabolite synthesis, and DNA repair, respectively. Additionally, PF0624, previously annotated as a hypothetical protein, was identified as a putative histone motif-containing protein. Experimental evidence suggests that PF0624 may contribute to chromatin formation via archaeal histones in P. furiosus. In summary, our findings reveal that P. furiosus responds to acute heat shock by maintaining protein stability, suppressing translation, limiting genomic damage, and potentially compacting genomic DNA into archaeal chromatin.