Consumption of clinically relevant cannabidiol doses during pregnancy alters postnatal behavior in offspring
Consumption of clinically relevant cannabidiol doses during pregnancy alters postnatal behavior in offspring
Gomez Wulschner, L.; Chang, V. N.; Oh, W. C.; Bates, E. A.
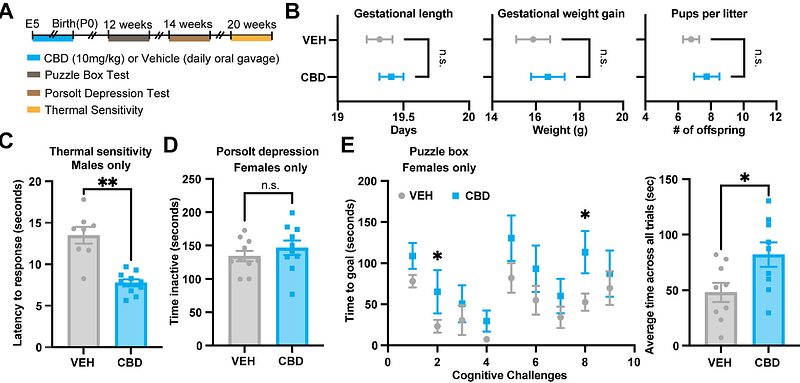
AbstractPregnant people use cannabidiol (CBD), a non-psychoactive cannabinoid of cannabis, due to its perceived safety and to treat side-effects such as nausea, insomnia, pain, and anxiety. However, CBD crosses the placenta and accumulates in the fetal brain, where it can activate or repress the function of several molecular targets that are important for brain development. While consumption of high doses of CBD during pregnancy have been shown to disrupt offspring neurodevelopment and postnatal behavior, lower, more clinically relevant doses have not been assessed. Here, we show that oral consumption of 10 mg/kg/day CBD during pregnancy increases thermal pain sensitivity in exposed male mouse offspring. Additionally, we find that the same dose impairs cognition and reduces excitability of the prefrontal cortex in exposed female mouse offspring. These data show that lower doses of CBD consumption during pregnancy can impair fetal brain development and postnatal behavior.