Optimized optogenetic anti-CRISPR for endogenous gene regulation in Drosophila
Optimized optogenetic anti-CRISPR for endogenous gene regulation in Drosophila
Ramongolalaina, C.; Jia, Y.; Zhang, E.; Pastor-Pareja, J. C.
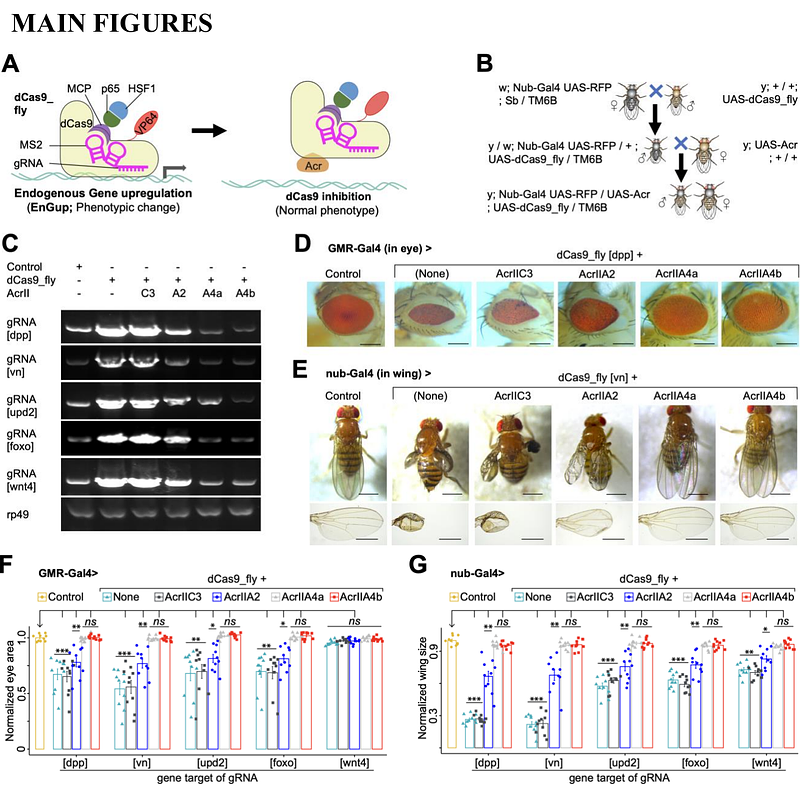
AbstractOptogenetic tools, whose engineering requires a structural understanding of the target proteins, are attractive approaches for achieving endogenous gene regulation under minimally invasive conditions. To build an optogenetic system for controlling endogenous gene expression, we first identified Anti-CRISPR (Acr) proteins that can inhibit CRISPRa-mediated transcriptional activation in Drosophila. Next, we inserted optogenetic protein LOV2 in these Acrs, tested for their ability to optogenetically modulate endogenous gene upregulation (EnGup) through the CRISPRa-based flySAM system in Drosophila, and found that the photoswitchability of these prototypes was weak. Hence, we engineered an Acr-LOV2 fusion module with refined length of intrinsically disordered and ordered regions (IDR and IOR) and optimized LOV2. This optimized variant, whose application yielded new findings in vivo, was significantly more sensitive for EnGup under blue light than the prototypes. In short, not only does this work introduce the application of Acr proteins in an animal model, but it also provides insights for in vivo characterization of the IDR and the IOR of these small-sized proteins. Together, these findings establish a robust optogenetic toolbox for precise, light-sensitive endogenous gene regulation in Drosophila.