Binding of extracellular vesicles to stretched von Willebrand factor promotes platelet activation
Binding of extracellular vesicles to stretched von Willebrand factor promotes platelet activation
Wang, Y.; Liu, X.; Downar, T.; Topuz, A.; Bauer, A. T.; Brenna, S.; Bendas, G.; Puig, B.; Schneider, S. W.; Fedosov, D.; Gorzelanny, C.
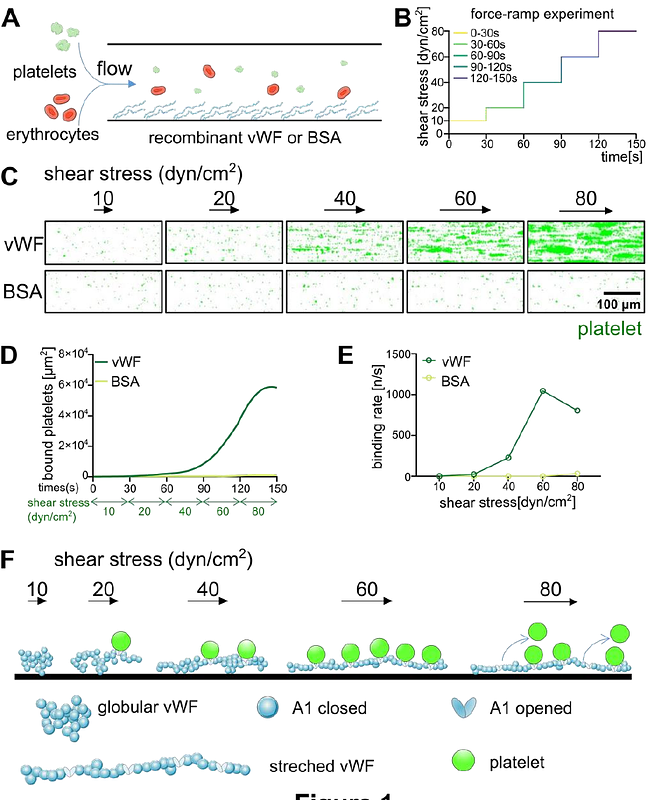
AbstractVon Willebrand factor (vWF), promoting platelet aggregation in various diseases such as COVID-19, malaria and cancer, is a huge multimeric glycoprotein. This extraordinary size makes vWF a unique shear stress sensing molecule. Below a critical shear stress, vWF is in a globular conformation that prevents platelet binding. Above the critical shear stress, vWF is stretched into platelet accessible fibers. Although previous studies have suggested that leukocytes or cancer cells can bind to vWF fibers, acting forces and the likelihood of cell adhesion has remained largely unexplored. Here, we report that vWF is a size-selective protein that prefers to interact with objects smaller than 4 m in diameter. Consistently, tumor cell-derived extracellular vesicles (EVs) were able to interact with vWF in parallel to platelets. Although whole tumor cells under flow were unable to bind to vWF per se, binding of EVs and platelets along the vWF fiber promoted platelet aggregation, which in turn entrapped circulating tumor cells. In conclusion, our study highlights the shear-sensitive nature of vWF and its ability to bring EVs and platelets together to enhance coagulation. While EVs-vWF-platelet aggregates may serve as novel biomarkers, their therapeutic disruption may prevent hypercoagulation in disease.