Sialylated CD43 is a glyco-immune checkpoint for macrophage phagocytosis
Sialylated CD43 is a glyco-immune checkpoint for macrophage phagocytosis
Chung, J.; Vallurupalli, M.; Noel, S.; Schor, G.; Liu, Y.; Nobrega, C.; Perera, J.; Wrona, E.; Hu, M.; Lin, Y.; Wu, D. w.; Saberi, M.; Scapozza, I.; Cruickshank, A.; Woods, E. C.; Chuong, C. L.; Birocchi, F.; Kammula, A.; Avila, O. I.; Kocak, M.; Doench, J. G.; Procter, D.; Thornton, L.; Brunner, A. M.; Winer, E.; DeAngelo, D. J.; Garcia, J. S.; Stone, R. M.; Jenkins, R. W.; Maus, M. V.; Graubert, T. A.; Yates, K. B.; Golub, T. R.; Manguso, R. T.
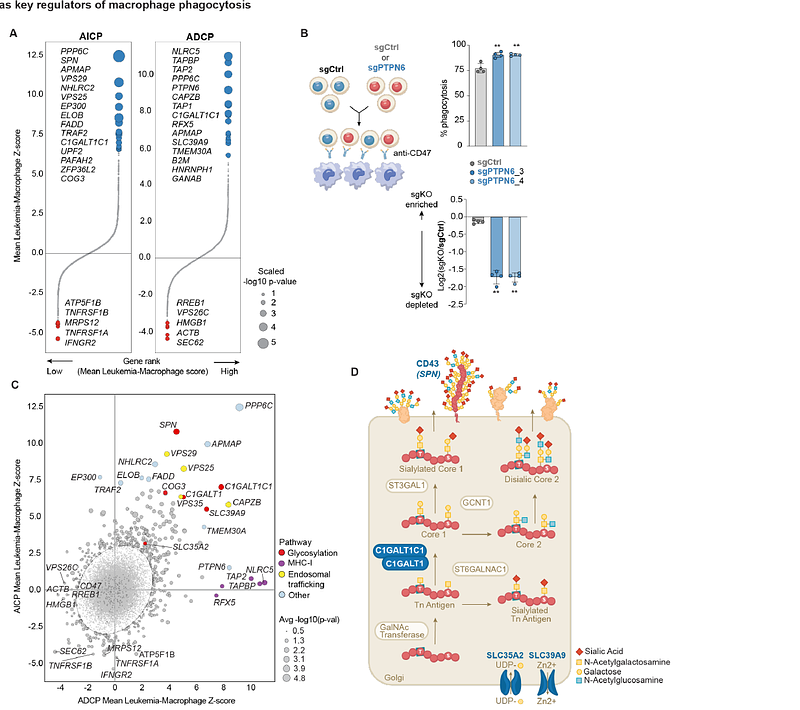
AbstractMacrophages in the tumor microenvironment exert potent anti-tumorigenic activity through phagocytosis. Yet therapeutics that enhance macrophage phagocytosis have not improved outcomes in clinical trials for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). To systematically identify regulators of phagocytosis, we performed genome-scale CRISPR knockout screens in human leukemia cells co-cultured with human monocyte-derived macrophages. Surprisingly, we found that whereas the classic \'\'don\'t eat me\'\' signal CD47 inhibited mouse macrophages, it did not inhibit phagocytosis by human macrophages. In contrast, the O-linked glycosylation and sialylation pathways were strong negative regulators of phagocytosis. In AML, the cell surface O-linked glycoprotein CD43 was the major effector of the O-linked glycosylation and sialylation pathways. Genetic deletion or antibody blockade of CD43 enhanced macrophage phagocytosis. This work highlights the importance of using human platforms to identify immune checkpoints, and nominates CD43 as a glyco-immune regulator of human macrophage phagocytosis.