MEK-dependent bioenergetic demand drives terminal CD8+ T cell exhaustion
MEK-dependent bioenergetic demand drives terminal CD8+ T cell exhaustion
Mitra, T.; Rahman, J.; Hwee, M.; Jesus Faustino Ramos, R. J.; Liu, H.; Hartman, T.; Cross, J.; de Jesus, M.; Huse, M.; Longo, V.; Zanzonico, P.; Vardhana, S. A.
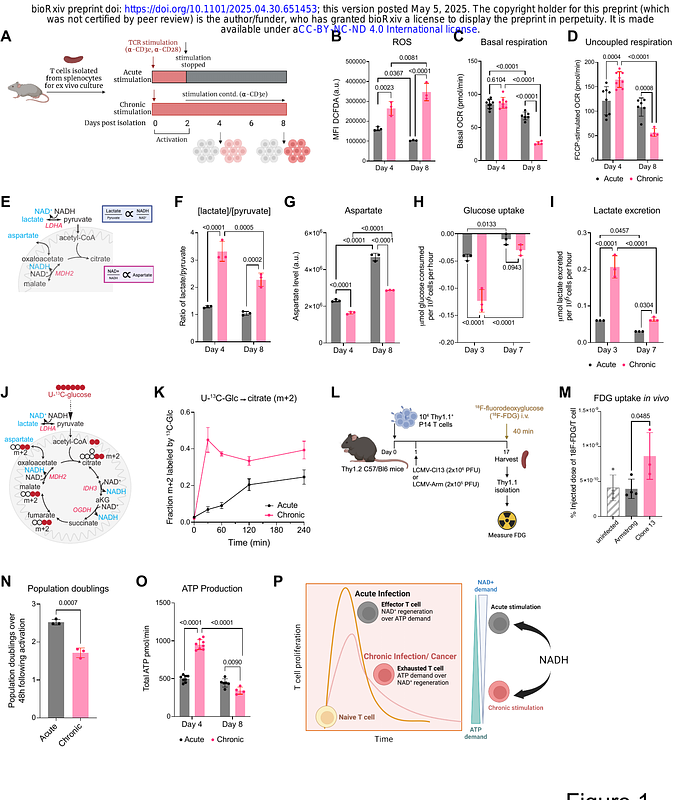
AbstractLoss of mitochondrial function contributes to CD8+ T cell dysfunction during persistent antigen encounter. How chronic antigen leads to this metabolic dysfunction remains unclear. Here, we show that TCR-dependent mitochondrial NADH accumulation drives production of ROS, ultimately leading to mitochondrial dysfunction. Among TCR-dependent proximal signaling components, MEK inhibition uniquely reduced nutrient uptake and mitochondrial NADH accumulation while increasing proliferation. As a result, MEK inhibition during chronic TCR stimulation reduced terminal T cell exhaustion. Mechanistically, we found that chronic MEK activation in T cells drove ATP demand by increasing global protein synthesis rates in vitro and in vivo. MEK inhibition reversed chronic TCR stimulation-driven increases in RNA polymerase II CTD phosphorylation, reducing transcription rates at effector- and terminal-exhaustion associated genes while maintaining transcription of memory-associated genes. These findings establish MEK-dependent metabolic demand as a driver of T cell exhaustion and elucidate the role of MEK inhibition in enhancing immunotherapy efficacy.