Liquid-phase determination of Arabidopsis respiration and photosynthesis using Clark-type O2 electrodes
Liquid-phase determination of Arabidopsis respiration and photosynthesis using Clark-type O2 electrodes
Sena, F.; Couture, C.; Berais-Rubio, A.; Millar, A. H.; Signorelli, S.
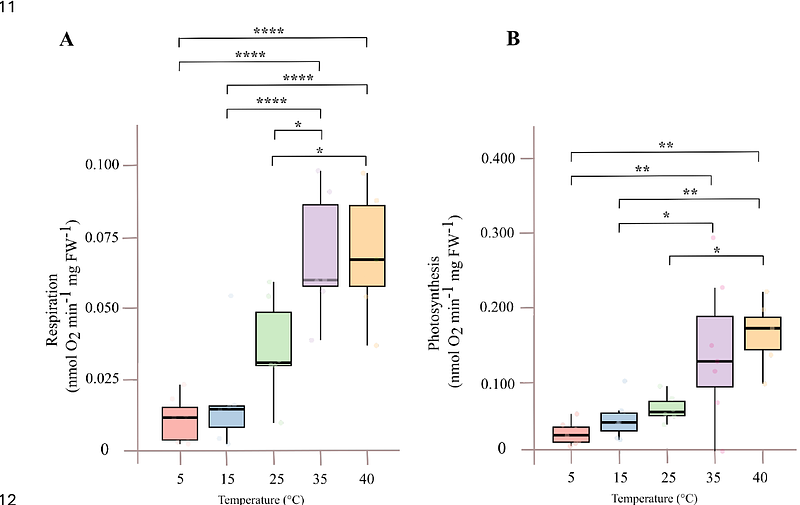
AbstractPhotosynthesis and respiration are fundamental metabolic processes in plants, tightly connected through shared substrates, energy dynamics, and redox balance. Arabidopsis is the key genetic model for plants but monitoring these sorts of physiological processes presents significant challenges using traditional gas-exchange or fluorescence-based techniques due to the small size of intact Arabidopsis thaliana (arabidopsis) seedlings. Here, we validate and characterize the use of Clark-type oxygen electrodes, specifically the Hansatech Oxytherm+P system, to quantify both photosynthetic and respiratory activity in intact arabidopsis seedlings. By monitoring oxygen evolution in dark and light phases, we demonstrate that oxygen consumption and production correspond to mitochondrial respiration and photosynthesis, respectively. These processes were modulated by tissue biomass, light intensity, developmental stage, and stress conditions. Specific inhibitors such as potassium cyanide and paraquat confirmed that the recorded changes in oxygen concentrations reflected mitochondrial cytochrome oxidase activity and photosystem electron transport-dependent oxygen production, respectively. Moreover, oxygen evolution increased significantly with bicarbonate supplementation, validating the system\'s sensitivity to carbon fixation. We further showed that photosynthetic activity measured with this method correlates with a quantitative green index and responds dynamically to de-etiolation, abiotic stress (salt, osmotic, oxidative), and temperature shifts. Our study lays the groundwork for measuring photosynthesis based on oxygen evolution and respiration in arabidopsis knockout mutants, CRISPR lines, overexpression lines and ecotypes using Clark-type oxygen electrodes and highlights key considerations and limitations to consider when applying this approach. This platform could also be adapted for many other small tissue plant samples.