Fluid shear stress promotes glial-mediated neurotoxicity in vitro via purinergic signaling
Fluid shear stress promotes glial-mediated neurotoxicity in vitro via purinergic signaling
Tenney, S.; Streilein, C.; Bermudez, A.; Keller, Z.; Cornelison, R. C.
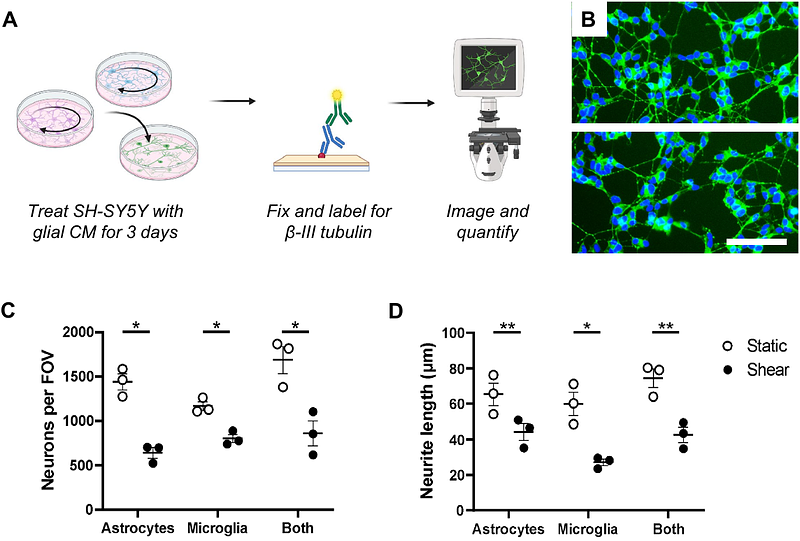
AbstractInterstitial fluid flow plays a critical role in maintaining function and homeostasis in neural tissue, and dysregulation of this flow due to injury or disease results in mechanical stress that is associated with several neuropathologies, including traumatic brain injury, ischemic stroke, and glioma. Glial cells such as astrocytes and microglia are known to respond to mechanical forces like fluid shear stress but the impact of this stress on their functionality and any subsequent impact on neurons remains poorly defined. To investigate how pathologically high fluid shear stress modulates astrocyte and microglia function and to determine whether glial responses to fluid shear influence neuronal survival and morphology, we applied low pathological levels of fluid shear stress (0.1 dynes/cm2) to cultured human astrocytes and microglia and assessed functional changes including metabolic activity, metabolite release, lipid droplet accumulation, and phagocytic activity. Conditioned media from these glia were then applied to differentiated SH-SY5Y neurons to evaluate effects on cell survival and neurite outgrowth. We then focused on identifying the soluble factor mediating the observed neurotoxicity. We found that fluid shear stress promotes distinct functional responses in astrocytes and microglia, including increased metabolic activity in astrocytes, increased lipid droplet accumulation in microglia, and heightened release of extracellular ATP in both cell types. Exposure to shear-conditioned glial media significantly reduced neuronal survival and neurite length. This neurotoxic effect was abolished by activated charcoal filtration but not by boiling and was prevented through P2X7 receptor inhibition in neurons, suggesting extracellular ATP as a causative agent. These findings indicate that high fluid shear stress promotes glial-mediated neurotoxicity via purinergic signaling. This study helps to characterize glial-neuronal mechanobiological interaction in the context of neuropathology and provides support for targeting purinergic signaling pathways as a therapeutic approach for neuropathologies associated with altered interstitial fluid flow.