An intrinsically disordered region of histone demethylase KDM5A activates catalysis through interactions with the nucleosomal acidic patch and DNA
An intrinsically disordered region of histone demethylase KDM5A activates catalysis through interactions with the nucleosomal acidic patch and DNA
Palla, A. M.; Lin, C.-C.; Trnka, M. J.; Leao, E. M.; Petronikolou, N.; Burlingame, A. L.; McGinty, R. K.; Fujimori, D. G.
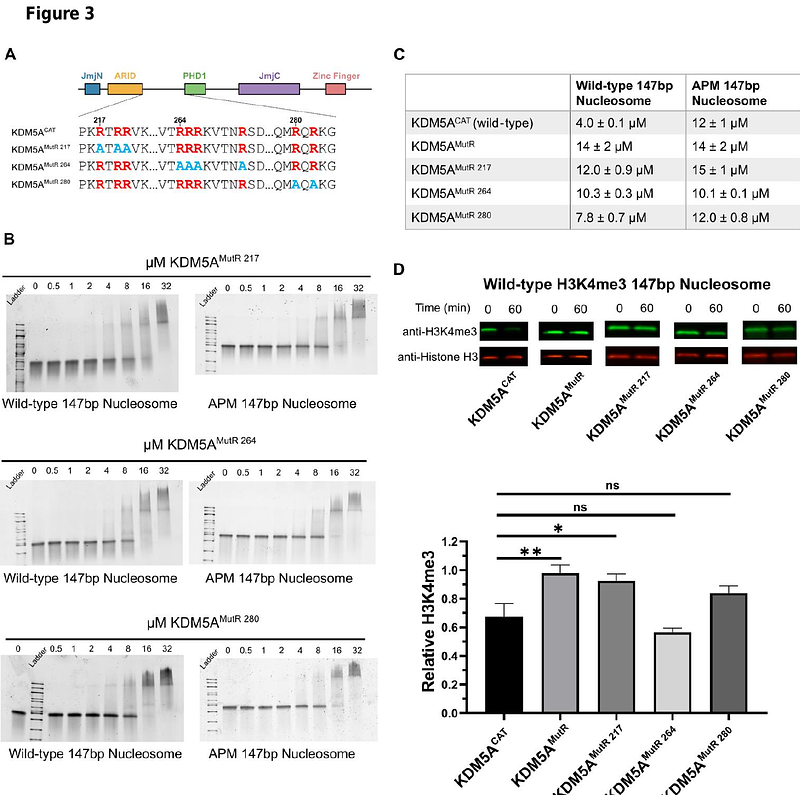
AbstractLysine demethylase 5A (KDM5A) plays a key role in the regulation of chromatin accessibility by catalyzing the removal of trimethyl marks on histone H3K4 (H3K4me3). KDM5A is also an oncogenic driver, with overexpression of KDM5A observed in various cancers, including breast, lung, and ovarian cancer. Past studies have characterized the functions of KDM5A domains, including KDM5A interactions with the histone H3 tail, but have yet to identify the broader mechanisms that drive KDM5A binding to the nucleosome. Through investigation of binding and catalysis on nucleosome substrates, we uncovered multivalent interactions of KDM5A with the H2A/H2B acidic patch and DNA that play crucial roles in the regulation of catalytic activity. We also identified an intrinsically disordered region (IDR) containing bifunctional arginine-rich motifs capable of binding to both the histone H2A/H2B acidic patch and nucleosomal DNA that is necessary for catalysis on nucleosome substrates. Our findings both elucidate previously unknown mechanisms that regulate KDM5A catalytic activity and reveal the ability of an IDR to engage in multiple interactions with chromatin.