H2O2-induced astrocytic collagen triggers neuronal death via fucosylation-dependent glial barrier formation upon ischemic stroke
H2O2-induced astrocytic collagen triggers neuronal death via fucosylation-dependent glial barrier formation upon ischemic stroke
Lee, J.-H.; Hwang, I.-Y.; Jang, H. J.; Lim, J.; Jung, J.; Kim, M.; Lee, E.; Won, W.; Lee, E. H.; Kim, K. J.; Park, K. D.; Jung, W.; Lee, B.; Ryu, S.; Lee, C. J.
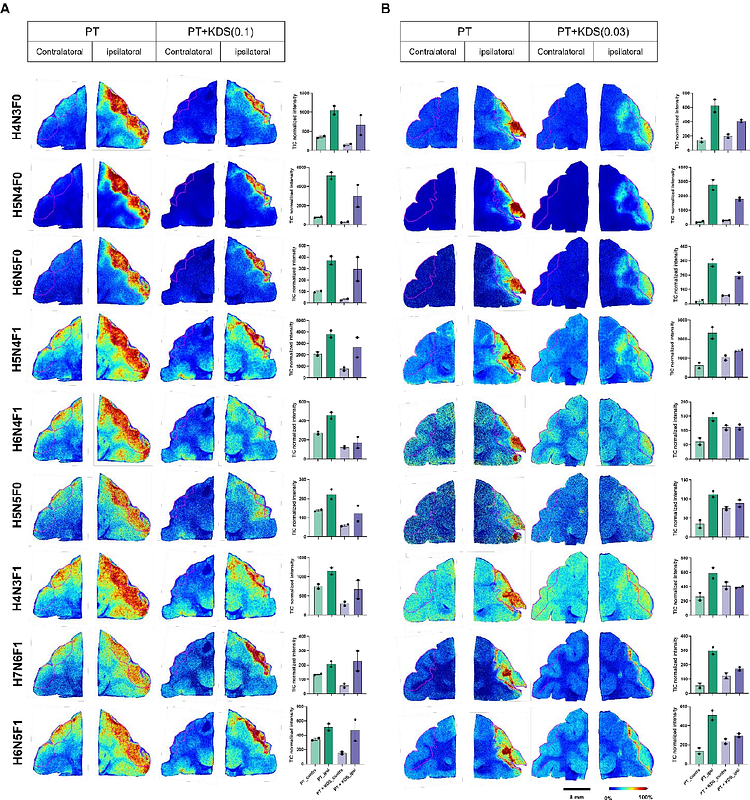
AbstractThe cascade of molecular and cellular events leading to neuronal death after a focal ischemic stroke remains enigmatic. Although astrocytes form glial barriers that may protect surrounding tissue, how barriers develop and contribute to neuronal death is unclear. Here, we show that H2O2 induces astrocytic type I collagen (COL1) production via miR-29 mediated post-transcriptional and fucosylation-dependent post-translational regulations, leading to integrin activation and neuronal death. In photothrombosis (PT)-induced cortical stroke model, PT triggered H2O2 surge, astrogliosis, glial barrier formation, COL1 expression, fibrotic scarring, altered N-glycosylation, neuronal loss, and neurological deficits. Remarkably, these effects were reversed by astrocyte-specific COL1 or FUT8 gene-silencing or treatment with KDS12025, an H2O2 decomposing peroxidase enhancer. KDS12025s neuroprotective effects were also recapitulated in non-human primate stroke model. These findings delineate a previously unrecognized astrocyte-driven mechanism in which oxidative stress induces COL1 production, promoting neuronal death, and position H2O2 and astrocytic COL1 and FUT8 as promising therapeutic targets for ischemic stroke.