Isomerized Aβ in the brain can distinguish the status of amyloidosis in the Alzheimer's Disease spectrum
Isomerized Aβ in the brain can distinguish the status of amyloidosis in the Alzheimer's Disease spectrum
Mukherjee, S.; Coyle, R.; Dubois, C.; Perez, K.; Mclean, C.; Masters, C.; Roberts, B. R.
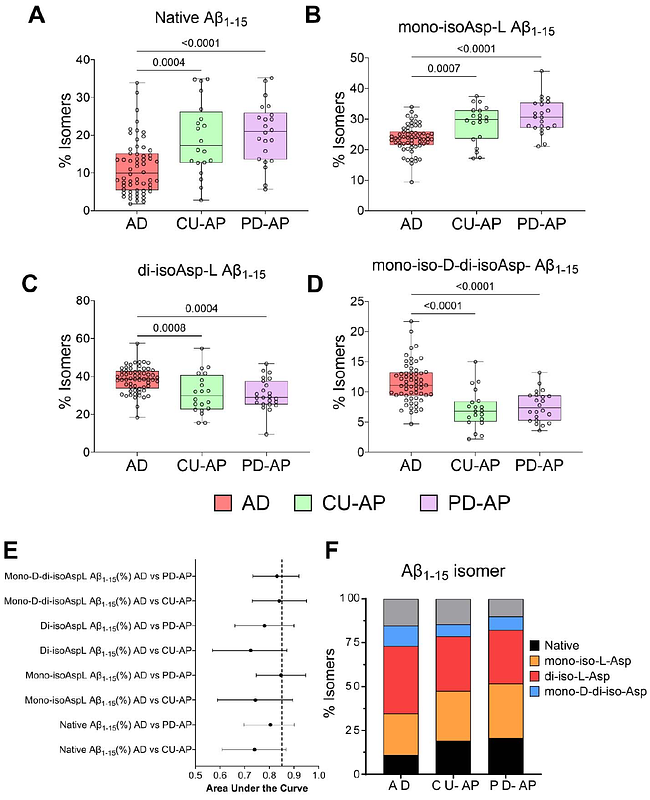
AbstractExtracellular amyloid plaques, the pathognomonic hallmark of Alzheimer\'s Disease (AD), are also observed in cognitively unimpaired subjects in the preclinical stages. Progressive accumulation of fibrillar amyloid-{beta} (A{beta}) as plaques and perivascular deposits occur two decades prior to clinical onset, making A{beta} a long-lived peptide. To characterize the amyloid plaques biochemically, both the A{beta} load as well the post-translational modifications (PTMs) could serve as markers for distinguishing the pre-clinical stage compared to later prodromal and clinical stages of AD. Recently, we described the presence of extensive isomerization of the A{beta} N-terminus in AD post-mortem brains that are significantly increased compared to the age-matched non-AD control brains with A{beta} aggregates in the frontal cortex. In this report, we used targeted mass spectrometry to conduct a quantitative analysis of the most common PTMs associated with A{beta} pyroglutamation, citrullination, N-terminal truncation (A{beta}4-x), C-terminal truncation (A{beta}42 and A{beta}40), and isomerization of aspartic acid residues (Asp-1 and Asp-7) in postmortem human brain tissue from pathologically negative (no A{beta} plaques) controls, controls with A{beta} plaques, Parkinson\'s disease (PD) with and without A{beta} accumulation/plaques and symptomatic AD. The AD cases contained statistically significant amounts of Asp-1and Asp-7 isomerized A{beta}1-15 (~ 90 %) compared to controls (preclinical AD) and PD brains with fibrillar A{beta} aggregates/deposits. We find that ratio of isomerized N-terminus A{beta} (A{beta}1-15) species in the brain detergent soluble pool differentiates older fibrillar A{beta} deposits in symptomatic AD brain compared to A{beta} deposits detected in preclinical AD and PD. Citrullinated A{beta}3pglu-15 was increased only in symptomatic AD, highlighting this A{beta} PTM is a unique feature of parenchymal plaques in advanced AD. Our results have implications for early therapeutic targeting of these modified species as well potential for better biofluid biomarker development for drug efficacy monitoring.