Ultra-High Resolution Solid-State NMR for High Molecular Weight Proteins on GHz-Class Spectrometers
Ultra-High Resolution Solid-State NMR for High Molecular Weight Proteins on GHz-Class Spectrometers
Wang, S.; Ravula, T.; Stringer, J. A.; Gor'kov, P.; Warmuth, O.; Williams, C.; Thome, A.; Mueller, L. J.; Rienstra, C. M.
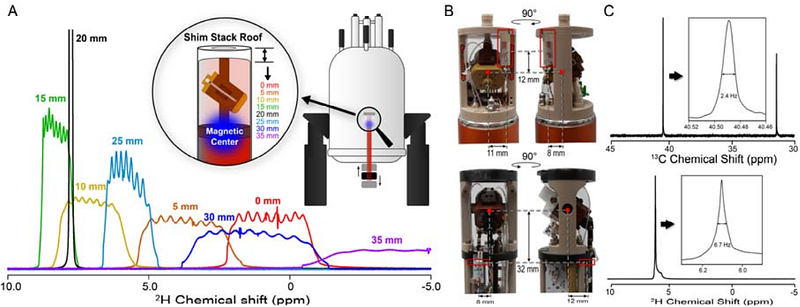
AbstractNMR spectroscopy is a powerful technique with broad impact across the physical and life sciences, and ultra-high field, GHz-class NMR spectrometers offer exceptional overall performance including superior resolution and sensitivity. While the resolution is fundamentally limited by molecular tumbling for solution NMR, solid-state NMR (SSNMR) is constrained only by instrumentation, making it well-suited for studying large and complex systems. To fully leverage UHF magnets for magic-angle-spinning SSNMR, it is essential to eliminate linebroadening arising from magnetic field drift and couplings among the nuclear spins. We address these challenges using an external 2H lock to compensate the field drift and Long-Observation-Window Band-Selective Homonuclear Decoupling (LOW-BASHD) to suppress 13C homonuclear couplings. We thereby achieve better than 0.2 ppm resolution in proteins up to 144 kDa, enabling unique site resolution for over 500 amide backbone pairs in 2D experiments. This exceeds the resolution available from solution NMR for large biological molecules, greatly expanding the potential of GHz-class NMR for research in life sciences.