Postoperative Stress Accelerates Atherosclerosis through Inflammatory Remodeling of the HDL Proteome and Impaired Reverse Cholesterol Transport
Postoperative Stress Accelerates Atherosclerosis through Inflammatory Remodeling of the HDL Proteome and Impaired Reverse Cholesterol Transport
Boucher, D. M.; Rochon, V.; Laval, T.; Lorant, V.; Carter, A.; Emerton, C.; Joyce, N.; Vinayak, N.; Scaffidi, M.; Auer, R. C.; Gordon, S. M.; Ouimet, M.
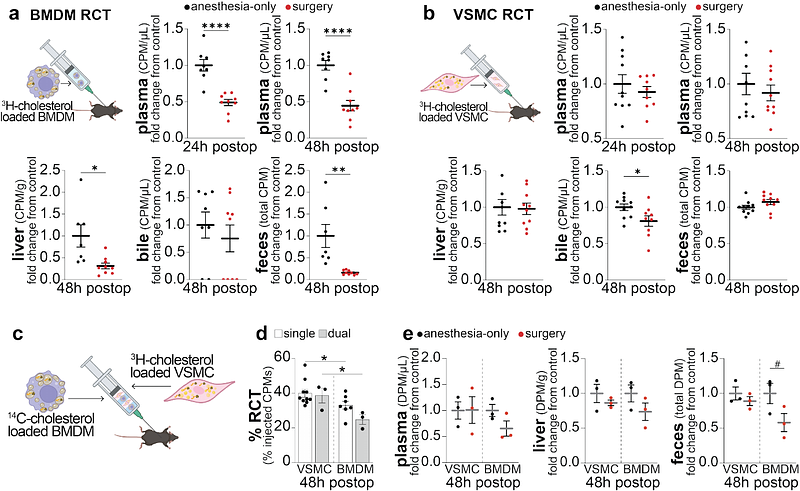
AbstractBACKGROUND: Over 10 million patients undergoing non-cardiac surgery annually experience major cardiovascular complications within 30 days, many due to destabilized atherosclerotic plaques. Reverse cholesterol transport (RCT), a key pathway for cholesterol removal by HDL and apoA-I, is critical in preventing plaque progression. While surgery-induced inflammation is known to impair HDL function, its effects on RCT and plaque stability remain unclear. METHODS: To isolate the impact of surgical inflammation, independent of blood loss, we developed an abdominal laparotomy model in apoE-/- mice on a Western diet, minimizing blood loss and avoiding perioperative blood sampling. We assessed plasma cholesterol efflux capacity, performed proteomic analysis of HDL, and analyzed atherosclerotic plaques for lipid content, perilipin-2 (PLIN2), cleaved-caspase-3 (c-Casp-3), and necrotic core expansion. A novel dual-label, dual-cell-type in vivo RCT model was developed to compare RCT from macrophage-derived (BMDMs) and vascular smooth muscle cells (VSMCs)-derived foam cells. Recombinant apoA-I (rApoA-I) was tested for therapeutic rescue of impaired RCT. RESULTS: Surgery significantly reduced RCT for at least 48 hours, paralleled by a drop in cholesterol efflux capacity and inflammatory remodeling of HDL, marked by elevated serum amyloid A (SAA1/2) and reduced apoA-I. Plaques showed a 1.6-fold increase in intracellular lipids and PLIN2 expression at 24 hours post-surgery, with elevated c-Casp-3 indicating lipid-driven apoptosis. Foam cell analysis revealed increased PLIN2 in both CD45+ (leukocyte) and CD45- (non-leukocyte) subtypes, with leukocyte foam cells expressing higher PLIN2. c-Casp-3+ apoptotic cells were predominantly PLIN2high and of both leukocytic and non-leukocytic origin. By day 15, the necrotic core area increased by 1.5-fold with sustained loss of plaque cellularity. Using our dual-cell-type RCT model, we found that surgery significantly impaired BMDM RCT in vivo, while VSMC RCT remained largely unaffected, highlighting foam cell subtype-specific vulnerability to surgical inflammation. These findings were mirrored in general surgery patients, whose postoperative plasma exhibited markedly reduced cholesterol efflux capacity. In mice, rApoA-I treatment partially restored RCT and reduced plaque lipid accumulation. CONCLUSIONS: Surgical inflammation acutely impairs HDL function and RCT, triggering lipid accumulation, foam cell apoptosis, and accelerated plaque destabilization independent of blood loss. Immediate restoration of apoA-I at the time of surgery, aiming to counteract the acute phase response, may offer a targeted strategy to reduce postoperative cardiovascular risk.