Hyper-Glycosylation as a Central Metabolic Driver of Alzheimers Disease
Hyper-Glycosylation as a Central Metabolic Driver of Alzheimers Disease
Hawkinson, T. r.; Liu, Z.; Ribas, R. A.; Medina, T.; Nielsen, R.; Clarke, H. A.; Ma, X.; Mueller, A.; Plasencia, A.; Shear, A.; Simpson, S.; Soto, C. M.; Sudderth, J.; Cai, F.; Cantrell, A. R.; Colpert, M. G.; Shedlock, C.; Wu, L.; Young, L. E. A.; Kooser, D. D.; chen, L.; Ryan, A. M.; Azadi, P.; Deberardinis, R. J.; Prokop, S.; Allison, D.; Huang, Y.; He, X.; Bian, J.; Vander Kooi, C. W.; Gentry, M. S.; Sun, R. C.
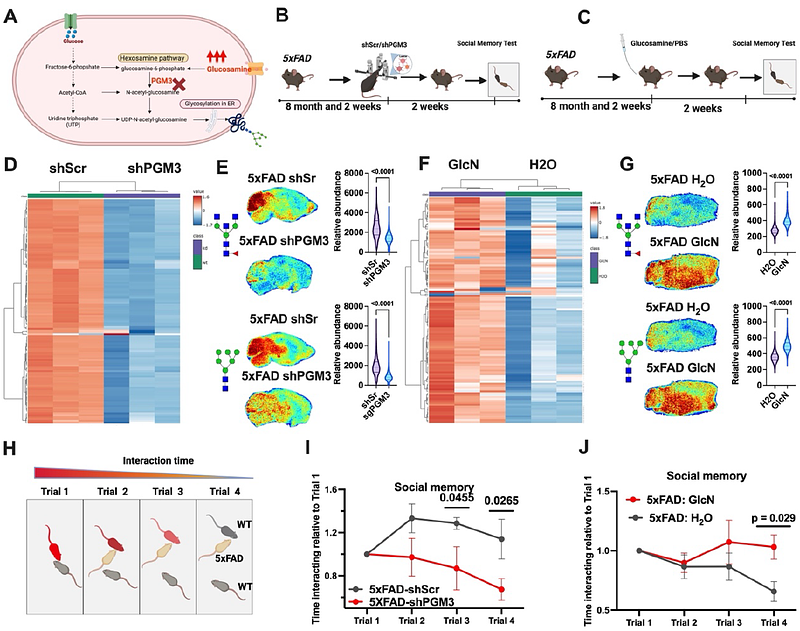
AbstractAlzheimer\'s disease (AD) is a neurodegenerative disorder characterized by devastating degenerative decline. Metabolic disruptions are widely observed, yet their involvement in the molecular etiology of AD remains underexplored. Utilizing spatial metabolomics, lipidomics, and glycomics in both mouse models and human post-mortem samples, we identified a hyper-glycosylation phenotype as a hallmark of AD. To investigate the underlying mechanisms and whether the observed effect was a driver of the observed decline, we developed an advanced spatial isotopic tracing pulse-chase method to study the dynamics of N-linked glycans. Our analysis revealed enhanced glycan biosynthesis in AD mouse models. Based on these findings, we performed genetic and dietary interventions to modulate glycan biosynthesis. Genetic knockdown of glycan biosynthetic enzymes ameliorated the hyper-glycosylation and improved cognitive and behavioral outcomes in AD mice. In contrast, oral glucosamine supplementation drove hyper-glycosylation and exacerbated cognitive and behavioral deficits. To assess the clinical relevance of these findings, we conducted a retrospective analysis of a large population of patients with mild cognitive impairment (MCI), AD, and Alzheimer\'s Disease Related Dementias (ADRD) stratified by glucosamine use, leveraging electronic health records. Consistently, glucosamine supplementation was associated with increased mortality in AD and ADRD patient cohorts, and significantly elevated progression from MCI to AD compared to age-matched controls. Collectively, our findings establish hyper-glycosylation as a pathological driver of AD and highlight glycan metabolism as an actional target in the fight against AD.