Functional and Structural Characterization of F1-ATPase with common ancestral core domains in stator ring
Functional and Structural Characterization of F1-ATPase with common ancestral core domains in stator ring
Suzuki, A. K.; Furukawa, R.; Sobti, M.; Brown, S. H. J.; Stewart, A. G.; Akanuma, S.; Ueno, H.; Noji, H.
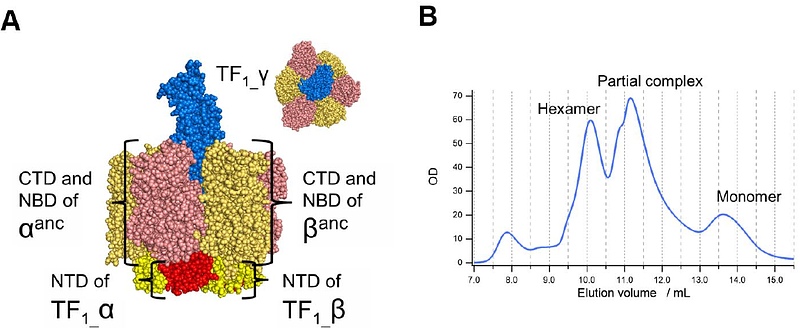
AbstractExtant F1-ATPases exhibit diverse rotational stepping behaviors--3-, 6-, or 9-step cycles--yet the evolutionary origin of these patterns remains unclear. Here, we used ancestral sequence reconstruction to infer the catalytic {beta} and non-catalytic subunits of a putative ancestral F1-ATPase. We then fused their functionally critical domains into the thermostable F1 from Bacillus PS3, yielding a stable chimeric enzyme. Cryo-EM revealed two distinct conformational states--binding and catalytic dwell states--separated by a ~34{degrees} rotation of the {gamma} subunit, suggesting a fundamental six-step mechanism akin to that of extant 6-stepping F1-ATPases. Single-molecule rotation assays with ATP and the slowly hydrolyzed ATP analog ATP{gamma}S demonstrated that the chimeric motor is intrinsically a 6-stepper, pausing at binding and catalytic dwell positions separated by 32.1{degrees}, although the binding dwell is significantly prolonged by an unknown mechanism. These findings indicate that F1-ATPase was originally a 6-stepper and diversified into 3-, 6- and 9-step forms in evolutional adaptation. Based on these results, we discuss plausible features of the entire FoF1 complex, along with potential physiological contexts in last universal common ancestor and related lineages.