Design and Characterisation of Photoactivatable and Lysine Reactive o-Nitrobenzyl Alcohol-Based Crosslinkers
Design and Characterisation of Photoactivatable and Lysine Reactive o-Nitrobenzyl Alcohol-Based Crosslinkers
Cahill, A.; Walko, M.; Fenton, B.; Ganji, S. R.; Herbert, A.; Radford, S.; Kapur, N.; Livingstone, K.; Wright, M.; Calabrese, A. N.
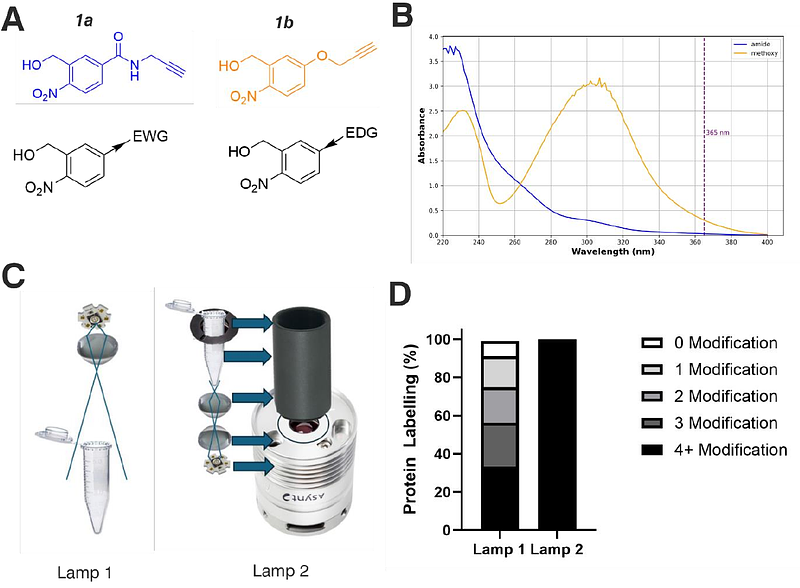
AbstractPhotoreactive groups are invaluable tools in structural proteomics, offering reagent-free activation and temporal control of protein labelling. However, traditional UV-activatable functional groups often produce unstable intermediates and diverse products, making these chemistries difficult to deploy at scale. In this study, we performed a systematic analysis of ortho-nitrobenzyl alcohol (oNBA) reactivity for integration into novel reagents for chemical crosslinking-mass spectrometry. oNBA photochemistry represents a promising alternative to traditional photoactivatable crosslinkers due to its unique specificity towards lysine residues. Here, we synthesised two molecules comprising oNBA functional groups with different substituents and assessed their labelling efficiency against a model protein. To ensure high labelling yields while maintaining a short irradiation time, we constructed a high power 365 nm irradiation device which improves the efficiency of oNBA photolysis. Our studies identified an amide-substituted probe that labels proteins with high efficiency. We next incorporated this optimised oNBA moiety into a homo-bifunctional crosslinker and a hetero-bifunctional crosslinker in combination with an NHS ester, which both resulted in high yields of crosslinked products. Our findings highlight that optimised oNBA-based reactive groups are viable UV-activated warheads that can deliver high labelling yields and efficient protein crosslinking, unlocking a wealth of potential structural proteomics applications.