DNA Actively Regulates the "Safety-Belt" Dynamics of Condensin during Loop Extrusion
DNA Actively Regulates the "Safety-Belt" Dynamics of Condensin during Loop Extrusion
Chen, J.; Feng, C.; Wang, Y.; Chu, X.
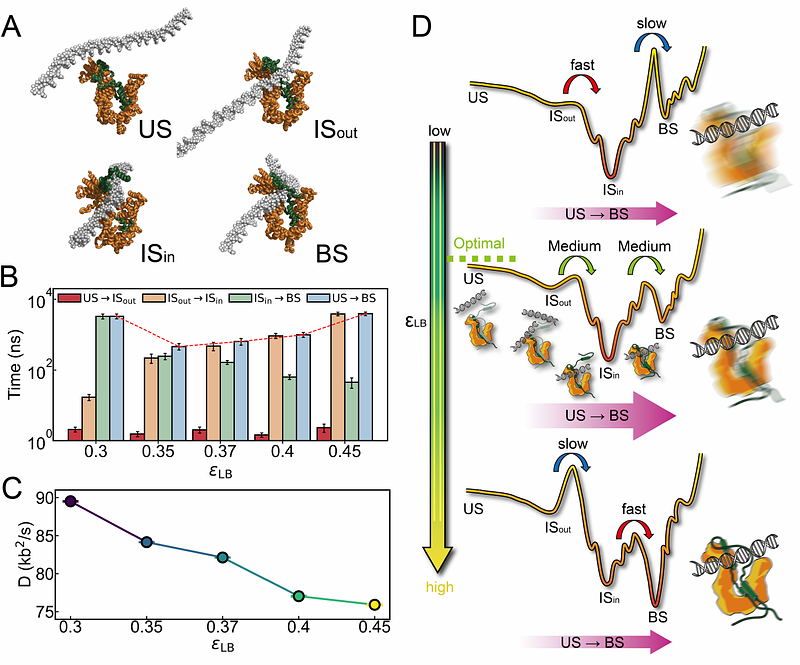
AbstractCondensin, a protein complex of the SMC (Structural Maintenance of Chromosomes) family, is essential for genome folding through its active DNA loop extrusion activity. Condensin contains a binding interface between its Ycg1 (HEAT-repeat) and Brn1 (kleisin) subunits that acts like a "safety belt" to trap DNA and prevent its dissociation during loop extrusion. The entrapment of DNA within the binding pocket of the SMC complex is crucial for ATPase activity and the asymmetric loop extrusion. However, the molecular mechanism underlying DNA entrapment remains unclear, hindering our understanding of how condensin functions at a molecular level. Here, we employ multiscale molecular dynamics simulations to reveal how DNA actively modulates condensin's safety-belt dynamics. Using all-atom simulations combined with AlphaFold3 predictions, we demonstrate that DNA binding markedly stabilizes the condensin safety belt, facilitating loop extrusion progression. Coarse-grained simulations capture the entire DNA entrapment process, highlighting an active regulatory role of DNA: DNA positioned outside the safety belt triggers its opening, whereas DNA within promotes closure, enhancing complex stability. Kinetic analyses further reveal that the rate-limiting step in DNA entrapment depends critically on the tightness of the safety belt. A loose safety belt makes the stable closure of its "latch" and "buckle" components rate-limiting, whereas a tighter safety belt shifts the barrier to initial DNA entry. Additionally, we find that latch-buckle interaction strength regulates condensin's sliding motion along DNA. Our findings provide a comprehensive molecular framework illustrating how DNA actively guides condensin's safety-belt dynamics, substantially advancing our mechanistic understanding of chromosome loop extrusion.