Uncovering Convergent Cell State Dynamics Across Divergent Genetic Perturbations Through Single-Cell High-Content CRISPR Screening
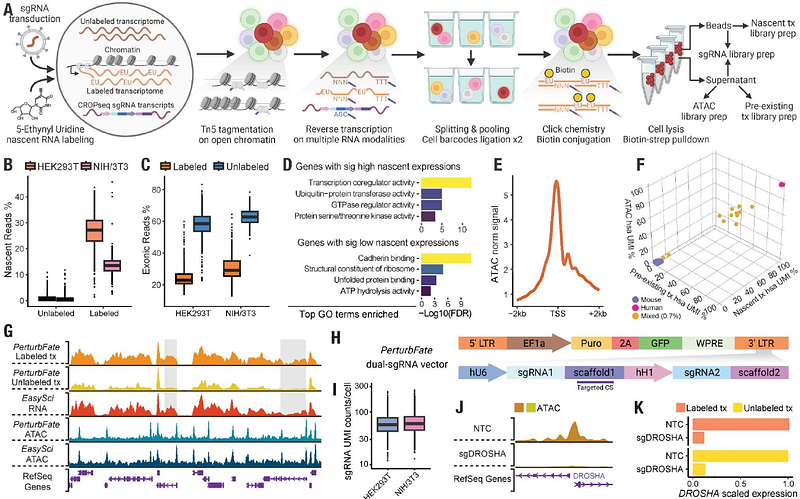
Uncovering Convergent Cell State Dynamics Across Divergent Genetic Perturbations Through Single-Cell High-Content CRISPR Screening
Xu, Z.; Lu, Z.; Ugurbil, A.; Abdulraouf, A.; Liao, A.; Zhang, J.; Zhou, W.; Cao, J.
AbstractHigh-throughput genomic studies have uncovered associations between diverse genetic alterations and disease phenotypes; however, elucidating how perturbations in functionally disparate genes give rise to convergent cellular states remains challenging. Here, we present PerturbFate, a high-throughput, cost-effective, combinatorial-indexing single-cell platform that enables systematic interrogation of massively parallel CRISPR perturbations across the full spectrum of gene regulation, from chromatin remodeling and nascent transcription to steady-state transcriptomic phenotypes. Using PerturbFate, we profiled over 300,000 cultured melanoma cells to characterize multi-modal phenotypic and gene regulatory responses to perturbations in more than 140 Vemurafenib resistance-associated genes. We uncovered a shared dedifferentiated cell state marked by convergent transcription factor (TF) activity signatures across diverse genetic perturbations. Combined inhibition of cooperative TF hubs effectively reversed cellular adaptation to Vemurafenib treatment. We further dissected phenotypic responses to perturbations in Mediator Complex components, linking module-specific biochemical properties to convergent gene activations. Together, we reveal common regulatory nodes that drive similar phenotypic outcomes across distinct genetic perturbations. We also delineate how perturbations in functionally unrelated genes reshape cell state. PerturbFate thus establishes a versatile platform for identifying key molecular regulators by anchoring multi-modal regulatory dynamics to disease-relevant phenotypes.