Env-antibody coevolution identifies B cell priming as the principal bottleneck to HIV-1 V2 apex broadly neutralizing antibody development
Env-antibody coevolution identifies B cell priming as the principal bottleneck to HIV-1 V2 apex broadly neutralizing antibody development
Habib, R.; Roark, R. S.; Li, H.; Connell, A. J.; Hogarty, M. P.; Wagh, K.; Wang, S.; Marchitto, L.; Skelly, A. N.; Carey, J. W.; Sowers, K. J.; Ayyanathan, K.; Plante, S. J.; Bibollet-Ruche, F.; Park, Y.; Agostino, C. J.; Singh, A.; Martella, C. L.; Lewis, E.; Lora, J.; Ding, W.; Campion, M. S.; Zhao, C.; Liu, W.; Li, Y.; Li, X.; Liang, B.; Chowdhury, R. R.; Amereh, K.; Van Itallie, E.; Sheng, Z.; Ghosh, A. R.; Bar, K. J.; Williams, W. B.; Wiehe, K.; Saunders, K. O.; Edwards, R. J.; Cain, D. W.; Lewis, M.; Batista, F. D.; Burton, D. R.; Andrabi, R.; Kulp, D. W.; Haynes, B. F.; Korber, B.; Shap
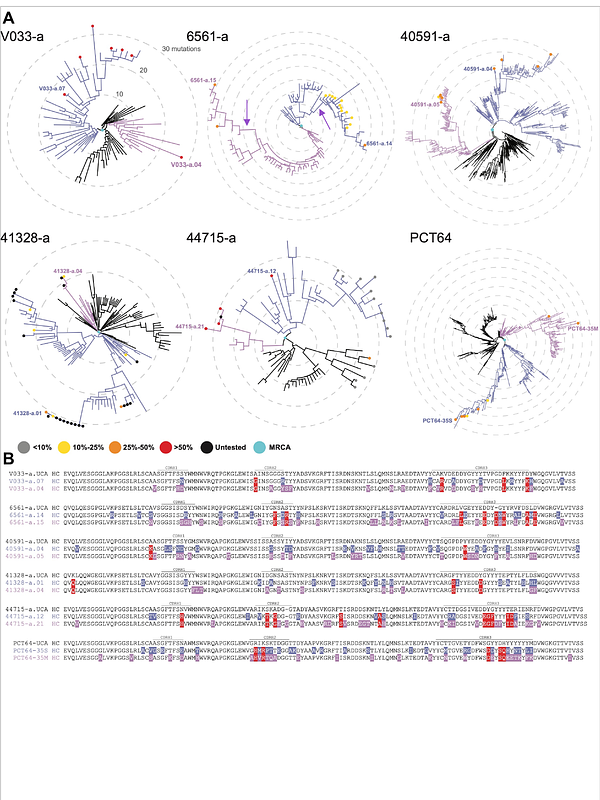
AbstractBroadly neutralizing antibodies (bNAbs) are rarely elicited during HIV-1 infection. To identify obstacles to bNAb development, we longitudinally studied 122 rhesus macaques infected by one of 16 different simian-human immunodeficiency viruses (SHIVs). We identified V2 apex as the most common bNAb target and a subset of Envs that preferentially elicited these antibodies. In 10 macaques, we delineated Env-antibody coevolution from B cell priming to bNAb development. Antibody phylogenies revealed permissive maturation pathways guided by evolving Envs that contained few mutations in or near the V2 apex C-strand, which were a sensitive indicator of apex-targeted responses. The absence of such mutations reflected a failure in bNAb priming. These results indicate that efficiency of B cell priming, and not complexities in Env-guided affinity maturation, is the primary obstacle to V2 apex bNAb elicitation in SHIV-infected macaques and identify specific HIV-1 Envs to advance as novel vaccine platforms.