Rational Molecular Design of Two-Photon Activated Temoporfin: A Computational Study for Advanced Photodynamic Therapy
Rational Molecular Design of Two-Photon Activated Temoporfin: A Computational Study for Advanced Photodynamic Therapy
Koca-Findik, B.; Uyar, E. S.; MONARI, A.; Catak, S.
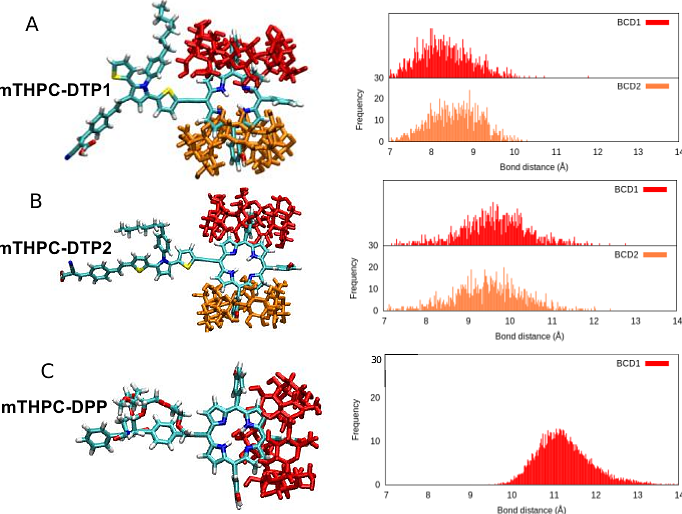
AbstractPhotodynamic therapy (PDT) is a promising, non-invasive cancer treatment that relies on the activation of photosensitizers (PS) by suitable light to produce cytotoxic reactive oxygen species. However, the efficiency of PDT is often hindered by the limited penetration of visible light into tissues, requiring the use of infrared activable PS. Furthermore, PS are usually prone to aggregation and present solubility issues limiting their bioavailability. In this study, we explore the functionalization of temoporfin (mTHPC), a clinically approved second-generation PS, with two-photon absorption (TPA) chromophores to enhance its efficiency in deep tissues. Three TPA-temoporfin conjugates (DTP1-mTHPC, DTP2-mTHPC, and DPP-mTHPC) have been designed and their properties have been investigated using a combination of quantum mechanics (QM), molecular dynamics (MD), and hybrid QM/MM simulations. Computational analysis revealed that the TPA cross-section ({sigma}) of the parent DTP moieties significantly increase when anchored to mTHPC, thus allowing efficient absorption in the near-infrared (NIR) region. Additionally, we have shown that their encapsulation with {beta}-cyclodextrins ({beta}-CDs) improved solubility and prevented aggregation without altering the optical properties of the PS. Simulations in a biological membrane model confirmed favorable interactions and localization of the candidate PDT agents within lipid bilayers, supporting their potential for enhanced clinical applications. This study demonstrates that rational molecular design can improve both the optical properties and the drug-delivery proficiency of temoporfin, paving the way for more effective deep-tissue PDT treatments.