Segmental Isotope Labelling of the Prion Protein: Identification of a Key Residue for Copper-Mediated Interdomain Structure
Segmental Isotope Labelling of the Prion Protein: Identification of a Key Residue for Copper-Mediated Interdomain Structure
Pavlovici, F. A.; Singewald, K.; Kaplan, S.; Chen, E.; Millhauser, G. L.
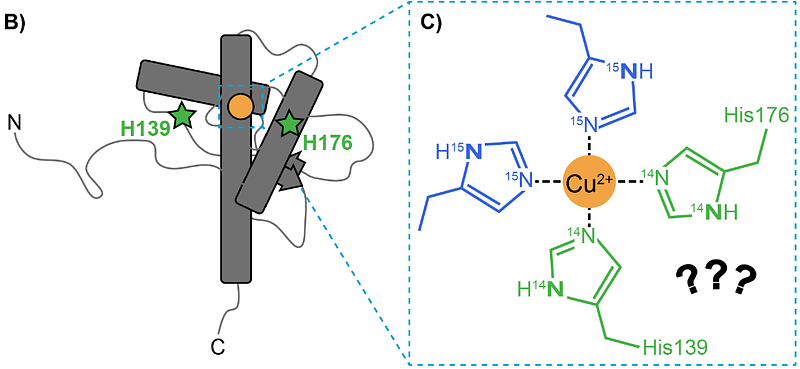
AbstractThe cellular prion protein is composed of two domains: a disordered N-terminal toxic effector domain and a three-helix C-terminal regulatory domain. Copper is thought to form a bridge between these two domains, inhibiting the protein\'s inherent neurotoxicity. However, the molecular details of how copper interacts with the C-terminal regulatory surface are unclear. To assess the potential role of conserved C-terminal His residues in copper coordination, we applied sortase-mediated ligation to create an expressed, murine prion protein with segmental 15N-labeling of the N-terminal domain. Pulsed EPR methods applied to a 1:1 protein:copper complex revealed both 14N and 15N couplings, consistent with simultaneous coordination of the two protein domains to the copper center. Mutagenesis studies localized C-terminal copper coordination to His176, present on the second alpha-helix. The cumulative EPR results reveal a copper coordination environment composed of three His residues from the protein\'s N-terminal domain, along with His176. The feasibility of these findings was tested with AlphaFold 3 simulations. These results further refine the molecular details of the prion protein autoregulation, emphasizing the critical role of its copper cofactor. Moreover, this interdisciplinary work demonstrates how sortase-mediated ligation combined with pulsed EPR sensitive to distinct nuclear spin systems provides a new strategy for assessing metal ion binding to proteins.