SMG7 and eIF4A constitute a homeostatic module controlling P-body condensation and function of Meiotic bodies
SMG7 and eIF4A constitute a homeostatic module controlling P-body condensation and function of Meiotic bodies
Cairo, A.; Shukla, N.; Kanavorova, S.; Skalak, J.; Mikulkova, P.; Vargova, A.; Potesil, D.; Zdrahal, Z.; Hejatko, J.; Riha, K.
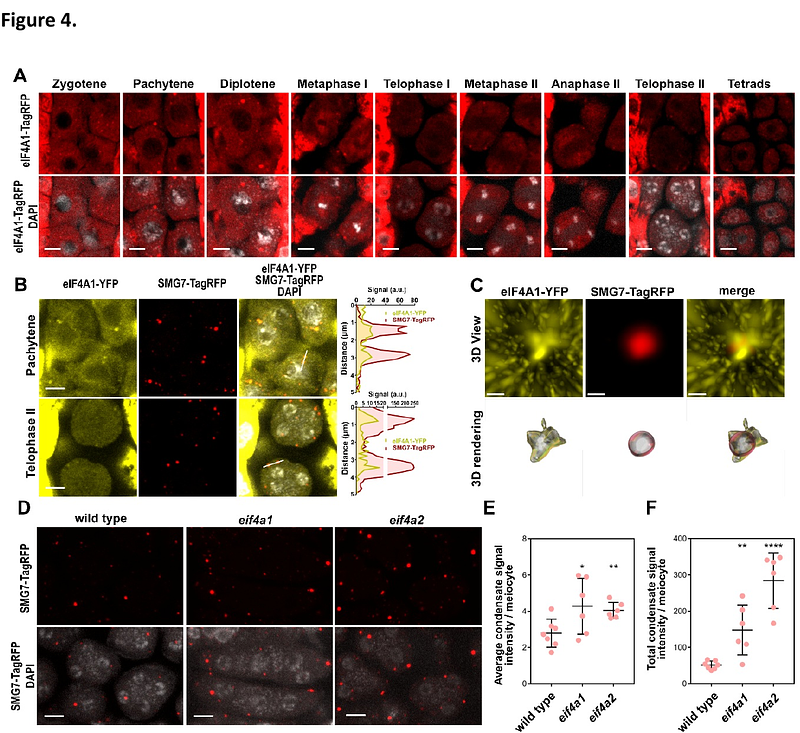
AbstractProcessing bodies (P-bodies) are ribonucleoprotein condensates that regulate RNA processing and storage. Although present constitutively in most cells, their size and composition dynamically change in response to developmental and environmental cues. However, mechanisms governing P-body assembly and remodelling remain poorly understood. Here we show that in Arabidopsis, SMG7 interacts with the eIF4A helicases and recruits them to P-bodies. eIF4As limit P-bodies condensation and also restrict stress granule (SG) formation under heat stress. We further identify meiotic (M-)bodies as composite RNP granules with a P-body core surrounded by a SG-like shell. The SMG7-eIF4A module regulates the recruitment of the meiosis-specific protein TDM1 into M-bodies, influencing meiotic exit and plant reproduction. Our findings suggest that SMG7 functions as an adaptor protein that recruits client proteins into P-bodies and, together with eIF4A, forms a regulatory module that regulates P-body composition and maintains their size homeostasis.