Single molecule imaging reveals extracellular signal-regulated kinases dependent signal responsive spatial regulation of translation in cardiomyocytes
Single molecule imaging reveals extracellular signal-regulated kinases dependent signal responsive spatial regulation of translation in cardiomyocytes
Erlich, I.; Douvdevany, G.; Haddad, R.; Prosser, B. L.; Kehat, I.
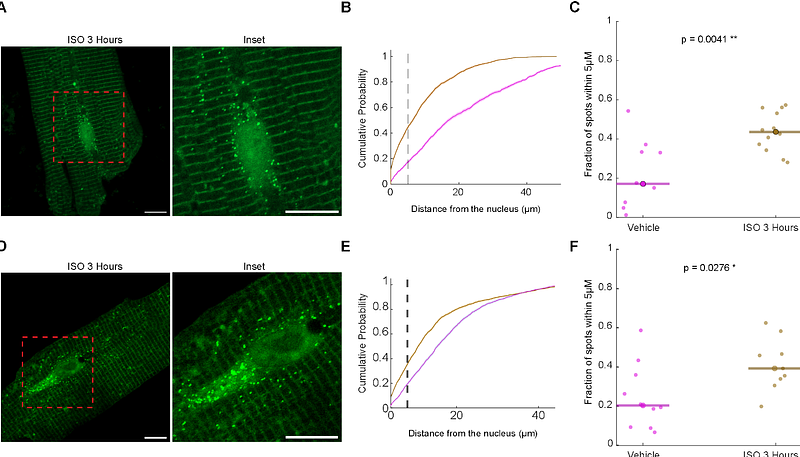
AbstractBackground: Translational control of gene expression is crucial in cardiomyocytes, particularly in response to hypertrophic stimuli. The extracellular signal-regulated kinases (ERK) pathway plays a key role in inducing cardiac hypertrophy and in regulating specific protein translation. However, it is unclear how the specificity is achieved, and the spatiotemporal dynamics of protein translation remain unexplored. Methods: We employed single-molecule imaging of nascent peptides (SINAPs) reporters to visualize and analyze the translation dynamics in single adult rat ventricular cardiomyocytes and tracked active translation sites at high spatiotemporal resolution. We also examined the effects of adrenergic stimulation and the role of the ERK pathway in translation localization. Results: Our findings revealed that translation sites are primarily localized near Z-lines in cardiomyocytes, with some sites being highly dynamic and moving during translation. The 3 untranslated regions did not significantly change the localization of translation. Many translation sites co-localized with microtubules, and their movement predominantly occurred along microtubular tracks. Adrenergic stimulation led to a transient shift in translation activity toward the peri-nuclear region, peaking at 12 hours and requiring ERK pathway activity for this localization change. Conclusions: Our high-resolution single-cell study demonstrates that protein translation in cardiomyocytes is dynamic and responsive to hypertrophic stimuli in an ERK-dependent manner. The localized translation mechanism allows cardiomyocytes to rapidly adapt to changing environments by preferentially translating mRNAs in the peri-nuclear region. These findings provide new insights into the spatial regulation of translation in cardiomyocytes and its role in cardiac hypertrophy.