Unacylated Ghrelin Counteracts Contractile and Mitochondrial Dysfunction in Cancer Cachexia
Unacylated Ghrelin Counteracts Contractile and Mitochondrial Dysfunction in Cancer Cachexia
Ahn, B.; Wanagat, J.; Cleary, C.; Ainsworth, H. C.; Kim, E.; Kim, H.
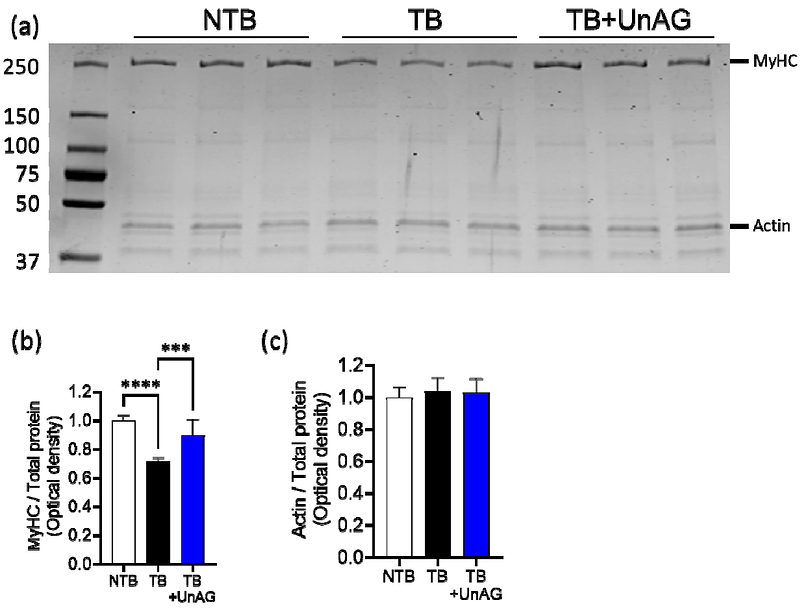
AbstractBackground Cancer cachexia is a complex metabolic syndrome that severely impacts patient mobility, treatment strategies, and quality of life. However, no treatments are available to mitigate the debilitating consequences of cancer cachexia. Unacylated ghrelin (UnAG), the main circulating form of ghrelin, enhances muscle growth and mitochondrial function in various diseases, but its effects in cancer cachexia remain to be tested. Methods Male C57Bl6/N mice were assigned to one of three treatment groups: non-tumor-bearing (NTB), tumor-bearing (TB), or tumor-bearing treated with unacylated ghrelin (TB+UnAG). Over four weeks, we monitored body weight, food intake, and tumor size. We assessed muscle mass, contractility, mitochondrial oxygen consumption rate (OCR), and reactive oxygen species (ROS) production. Proteomic analysis was performed to elucidate the downstream effects of UnAG. Cell culture assays were performed to measure the in vitro effects of cancer cell-secreted factors and UnAG on myoblasts. Results Gastrocnemius and quadriceps muscle masses were reduced by 20-30% in TB mice compared to NTB controls; however, UnAG treatment prevented approximately 50% of this loss. Beyond muscle mass, UnAG enhanced the isometric maximum specific force of the extensor digitorum longus by 70% in TB mice. This improvement in muscle quality was associated with preferential upregulation of myosin heavy chain expression in TB+UnAG mice. UnAG also increased mitochondrial OCR while reducing ROS production. Mitochondrial DNA (mtDNA) copy number, which was reduced in TB mice, was restored by UnAG, while the reduced mtDNA mutation frequency in TB mice was maintained with treatment, indicating improved mtDNA integrity. Consistent with enhanced mitochondrial function, treadmill running time was significantly increased in TB+UnAG mice. Proteomic analysis revealed that UnAG downregulated proteins associated with proteolysis, while normalizing antioxidant enzyme thioredoxin and proteins involved in calcium handling. Cancer cell-conditioned medium reduced myotube width in vitro, but UnAG treatment preserved myotube structure.. Conclusion UnAG protects against cancer cachexia by targeting multiple risk factors, including myosin heavy chain expression, mitochondrial bioenergetics, and modulation of protein degradation pathways.