Novel HIV-1 fusion peptide immunogens using glycan-engineered alphavirus-like particles
Novel HIV-1 fusion peptide immunogens using glycan-engineered alphavirus-like particles
Oh, S.; Gudipati, D. R.; Shi, W.; Zhao, P.; Wu, W.; Boyington, J. C.; Nariya, H. K.; McGhee, E. G.; Azzam, T.; Raghunathan, V.; Yang, C.; Yang, C.; Lee, C.; Kim, J. D.; Zhou, T.; Mascola, J. R.; Wells, L.; Kong, R.
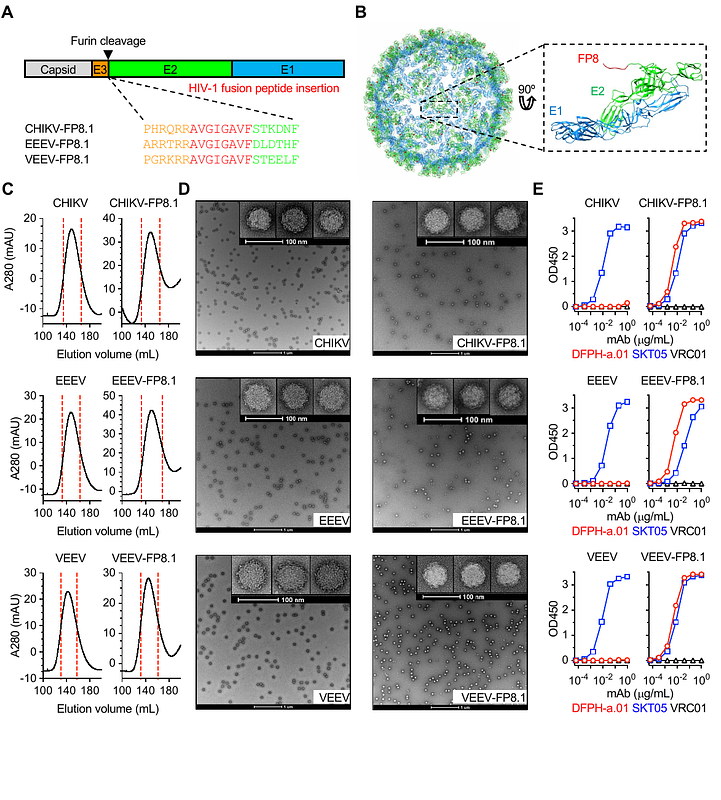
AbstractImmunofocusing on conserved, subdominant epitopes is critical for vaccines against highly diverse viruses such as HIV-1, influenza, and SARS-CoV-2. The eight-residue N-terminus of the HIV-1 fusion peptide (FP) is one such example of a promising yet small target. We developed new FP immunogens using three alphavirus-like particles (VLPs) and introduced additional glycans to mask shared carrier-specific epitopes. In two independent guinea pig studies, sequential immunization with heterologous carriers enhanced FP-directed antibody titers, which were further improved with glycan engineering. Separately, using diverse FP variants sharing the same N-terminal six amino acids increased neutralizing antibody titers. When combined, these two strategies led to higher FP-directed titers and, after Env trimer boosting, induced FP-directed neutralizing antibodies against multi-clade wild-type HIV-1 in nearly all animals. These findings established the importance of minimizing recurrent off-target epitopes across immunizations and support the engineered VLPs as a promising platform for peptide immunization.