A moss N-Acetyltransferase-MAPK protein controls 2D to 3D developmental transition via acetylation and phosphorylation changes
A moss N-Acetyltransferase-MAPK protein controls 2D to 3D developmental transition via acetylation and phosphorylation changes
de Luxan Hernandez, C.; Ammitsoe, T. J.; Kanne, J. V.; Stanimirovic, S.; Roux, M.; Weeks, Z.; Schutzbier, M.; Dürnberger, G.; Roitinger, E.; Zhang, L.; Spadiut, O.; Ishikawa, M.; Hasebe, M.; Moody, L.; Dagdas, Y.; Rodriguez, E.; Petersen, M.
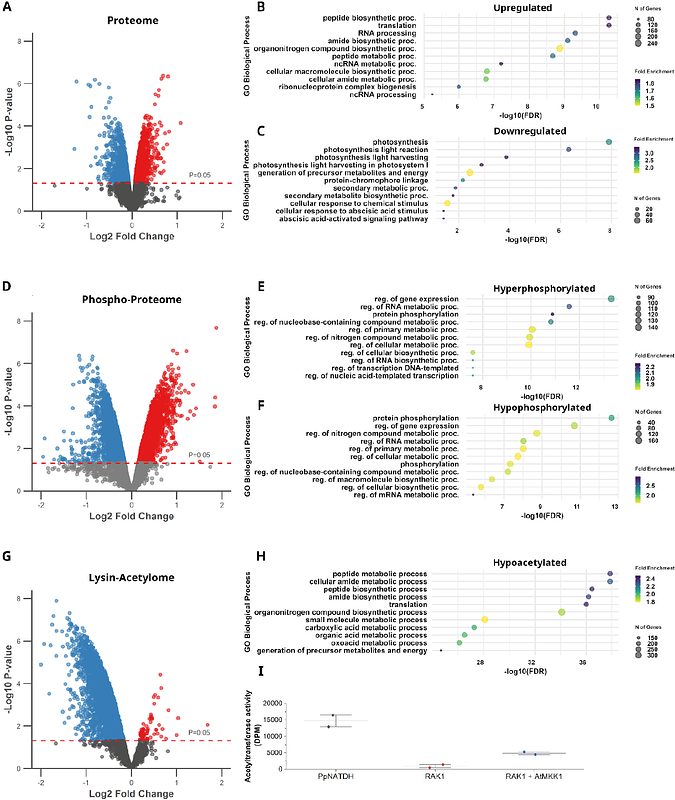
AbstractPost-translational modifications (PTMs) finetune plant responses to developmental and environmental cues by impacting protein activity, stability, localization and interaction landscape. In this study we identified a moss specific protein which combines two common PTMs: acetylation and phosphorylation. This protein originated from the fusion of a MAPK with an N-acetyltransferase, for which we named it Rosetta NATD-MAPK 1 (RAK1). Using biochemical methods, we demonstrated that RAK1 has acetyltransferase activity that is enhanced by activation of its MAPK domain. Phenotypical studies of rak1 knockout mutants revealed a role for RAK1 in the regulation of the 2D-to-3D growth transition. Through Mass Spectrometry we verified that defective 2D-to-3D transition in the mutants was caused by differentially regulated acetylation and phosphorylation events associated to metabolic reprogramming and 3D differentiation. Collectively, this study uncovers a previously unknown multidomain protein and provides insights into the interplay of PTMs during developmental reprogramming.