Interplay Between Intrinsically Disordered Proteins and Atomically Precise Gold Nanoclusters Modulates their Optical Properties
Interplay Between Intrinsically Disordered Proteins and Atomically Precise Gold Nanoclusters Modulates their Optical Properties
Rodriguez, S.; Kumanski, S.; Ayed, Z.; Fournet, A.; Bouanchaud, C.; Sagar, A.; Allemand, F.; Resch-Genger, U.; Cortes, J.; Sibille, N.; Chirot, F.; Wegner, K. D.; Antoine, R.; Le Guevel, X.; Bernado, P.
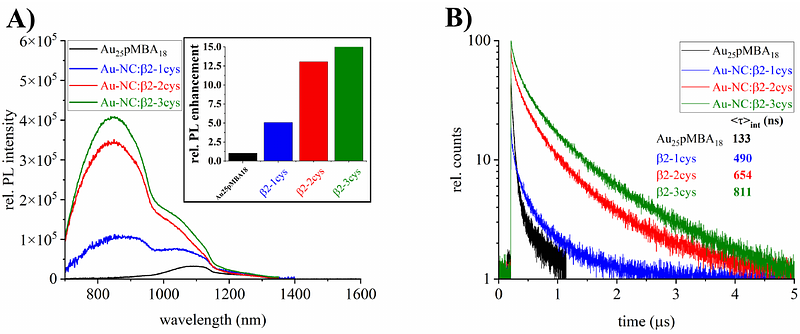
AbstractUnderstanding how structural and optical properties of metallic nanoclusters can be tuned by proteins is crucial for the use of these hybrid molecules in biomedical applications. The interaction of proteins with ultrasmall, atomically-precise gold nanoclusters (Au-NCs) has been mainly investigated in the context of structured proteins, while their behavior with intrinsically disordered proteins (IDPs) re-mains unexplored. This work examines the structural and optical properties of Au-NCs interacting with bioengineered IDPs containing up to three cysteines. We show that, by exploiting the conformational flexibility of cysteine-containing IDPs, we can anchor proteins to Au-NCs in a position-specific man-ner, leading to new bioconjugates with properties that differ from those of the individual components. We observed an up to 15-fold photoluminescence enhancement depending on the number of cysteines anchored. By combining mass spectrometry, small-angle X-ray scattering (SAXS), and computational modelling, the ensemble structures of nine bioconjugates with different stoichiometries were elucidat-ed, indicating their overall compactness. Our results suggest that the interface between these atomical-ly-precise species and the conformationally fluctuating protein is responsible for the optical properties of these nanobioconjugates. This research improves our understanding of Au-NC protein interactions, paving the way to novel nano-molecular hybrid conjugates with tunable properties for bioimaging and therapeutic applications.