Ivermectin binds to the allosteric site (site 2) and inhibits allosteric integrin activation by pro-inflammatory cytokines.
Ivermectin binds to the allosteric site (site 2) and inhibits allosteric integrin activation by pro-inflammatory cytokines.
Takada, Y. K.; Takada, Y.
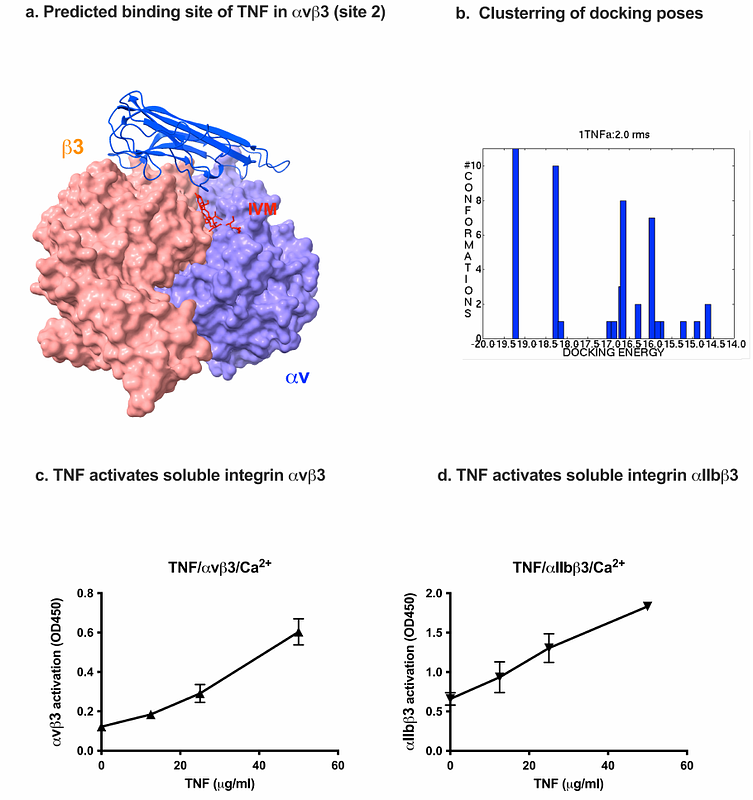
AbstractIvermectin is known to have anti-inflammatory properties, but the specifics of this action are unknown. We recently showed that multiple pro-inflammatory cytokines (e.g., FGF2, CCL5, CD40L) bind to the allosteric site (site 2) of integrins and activate them. 25-Hydroxycholesterol, a pro-inflammatory lipid mediator, is known to bind to site 2 and induce integrin activation and inflammatory signals (e.g., IL-6 and TNF secretion), suggesting that site 2 is critically involved in inflammation. We showed that TNF, a major pro-inflammatory cytokine, binds to site 2 and induces integrin activation, like other pro-inflammatory cytokines. We recently showed that two anti-inflammatory cytokines (FGF1 and NRG1) bind to site 2 and inhibit integrin activation by inflammatory cytokines. We hypothesized that ivermectin binds to site 2 but inhibits the integrin activation induced by inflammatory cytokines in a mechanism similar to anti-inflammatory cytokines (site 2 antagonists). Consistently, docking simulation predicts that ivermectin binds to site 2. We found that ivermectin suppressed the activation of soluble {beta}3 integrins by multiple pro-inflammatory cytokines (including TNF) in a dose-dependent manner in cell-free conditions, suggesting that ivermectin acts as an antagonist for site 2. This may be a potential mechanism of anti-inflammatory action of ivermectin.