Electron bifurcation arises from emergent features of multicofactor enzymes
Electron bifurcation arises from emergent features of multicofactor enzymes
Wojcik-Augustyn, A.; Bujnowicz, Łukasz; Osyczka, A.; Sarewicz, M.
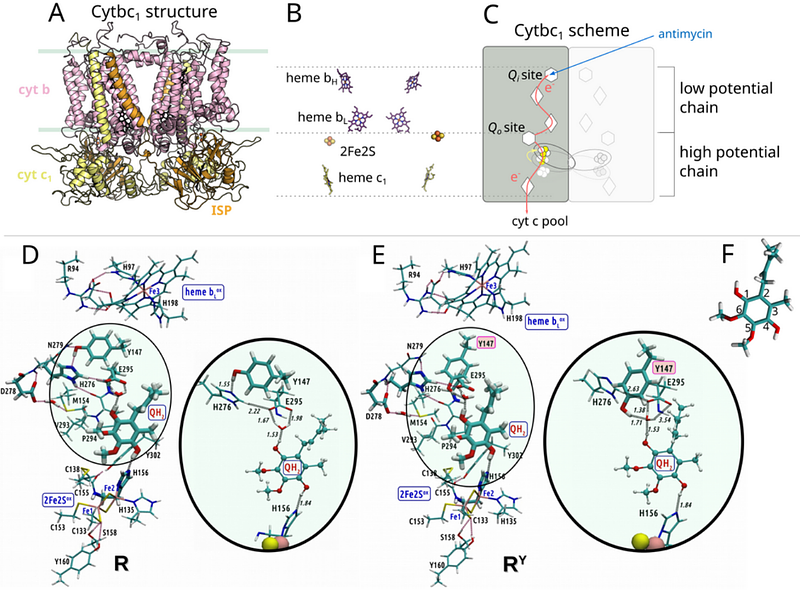
AbstractQuinone-based electron bifurcation (EB) catalyzed by cytochrome bc (cytbc) plays a critical role in energy conversion during respiration and photosynthesis. The canonical EB model (CEB), grounded in equilibrium redox potentials, dictates the order of initial EB steps with initial reduction of high-potential iron-sulfur cluster (2Fe2S) by quinol followed by reduction of low-potential heme bL (bL) by semiquinone. However, this concept falls short in explaining several experimental observations, including intermediate semiquinone spin-coupled to 2Fe2S and the absence of short-circuiting. We applied quantum mechanical calculations on large cluster models of cytbc, encompassing both 2Fe2S and bL, to investigate the early stages of EB. Our results reveal that EB is an emergent property of an integrated system of redox cofactors, where transient charge separations dynamically modulate electron affinities. In this system, electron transfer initiates preferentially toward bL, indicating a departure from the conventional sequence proposed by CEB. Based on this finding we introduce an EMBER (EMergent BL-first Electron Routing) mechanism of EB and show by electron paramagnetic resonance spectroscopy that predictions derived from this mechanism receive strong experimental validation. We argue that EMBER explains the observed stability of semiquinone coupled to 2Fe2S and suppression of energy wasting short-circuits without additional assumptions. It highlights the importance of dynamic electrostatic interactions in shaping electron transfer pathways in biological systems. In general, the concept of emergence inherent to EMBER offers a mechanistic framework applicable to a broad range of multi-cofactor redox enzymes beyond cytbc.