ATPγS unbiases kinesin
ATPγS unbiases kinesin
Karnawat, V.; Toleikis, A.; Molloy, J.; Carter, N.; Cross, R. A.
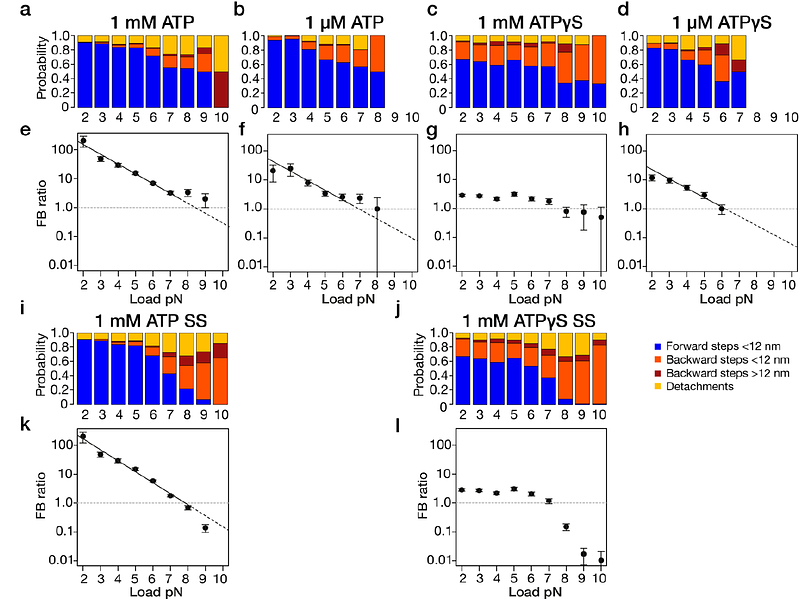
AbstractKinesin-1 microtubule motors are ATP-fuelled, twin-headed cargo transporters that step processively along microtubules, with a load-dependent directional bias. Here we show using single molecule optical trapping that 1 mM ATP{gamma}S, a slowly-hydrolysed analogue, substantially defeats the biasing mechanism, whereas 1 M ATP{gamma}S supports it. Our data argue that kinesin re-registers itself between steps into an Await-Isomerisation (AI) state that engenders both on-axis steps and off-axis missteps, and that missteps can be rescued. In the AI state, ATP or ATP{gamma}S is bound but neck-linker docking and nucleotide hydrolysis are inhibited. Load-dependent exit from the AI state establishes hydrolytic competence via catalytic site closure and coupled neck-linker docking, which guides the tethered head to its next on-axis site. By overpopulating the AI state, ATP{gamma}S reveals its pivotal role in the biasing mechanism, whose control logic maximises steered diffusion-to-capture of the leading kinesin-1 head under load, at the expense of futile nucleotide turnover.