Optimizing cardiac organoid culture to enhance maturation, viability, and cardiotoxicity assessments
Optimizing cardiac organoid culture to enhance maturation, viability, and cardiotoxicity assessments
Harihara, A.; Moshksayan, K.; Momtahan, N.; Ben-Yakar, A.; Zoldan, J.
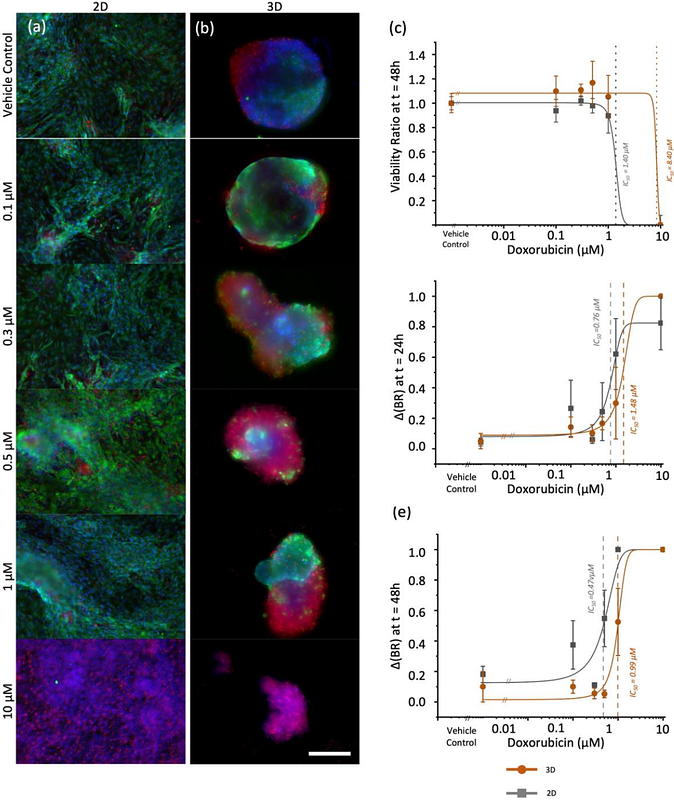
AbstractDevelopment of relevant, human induced pluripotent stem cell-derived cardiac organoids is essential to recapitulate myocardium physiology and functionality for assessment of drug-induced toxicity evaluations. However, the optimal conditions for culturing self-aggregating multicellular cardiac organoids are not well-elucidated, particularly the impact of noncardiomyocytes. In this study, we generated cardiac organoids at varying seeding densities to formulate organoids that meet or exceed the biological diffusion limit. We assessed their morphology, gene expression profiles, beating functionality, viability, and mitochondrial activity over time. Our results show that organoid sizes stabilize by seven days of culture, regardless of seeding density. However, organoids seeded with 20,000 cells retained an optimal cardiac signature that promotes cardiac maturity and minimizes fibrotic tendencies, especially when culturing longer than seven days. While all organoid populations maintained their beating functionalities, those seeded with 80,000 cells exhibited greater cell shedding and increased apoptosis at long term culture. In contrast, minimal apoptosis was observed in organoids seeded with 20,000 cells after seven days. Mitochondrial staining further revealed that organoids seeded with 20,000 cells consistently demonstrated higher metabolic activity. Taken together, organoids seeded with 20,000 cells and cultured for seven days yielded the healthiest morphology, transcriptional signature, and viability, while maintaining robust beating kinetics. Importantly, compared to 2D cultures, these optimized organoids demonstrate improved sensitivity to clinically relevant doxorubicin-induced cardiotoxicity, enabling more accurate dose-response evaluations that better reflect therapeutic conditions.