Identification of uranyl-binding proteins in Arabidopsis thaliana cells exposed to uranium: Insights from a metalloproteomic analysis and characterization of Glycine-Rich RNA-binding Protein 7 (GRP7)
Identification of uranyl-binding proteins in Arabidopsis thaliana cells exposed to uranium: Insights from a metalloproteomic analysis and characterization of Glycine-Rich RNA-binding Protein 7 (GRP7)
Revel, B. H.; Favier, A.; Martin-Laffon, J.; Vallet, A.; Przybyla-Toscano, J.; Brugiere, S.; Coute, Y.; Diemer, H.; Cianferani, S.; Rabilloud, T.; Bourguignon, J.; Brutscher, B.; Ravanel, S.; Alban, C.
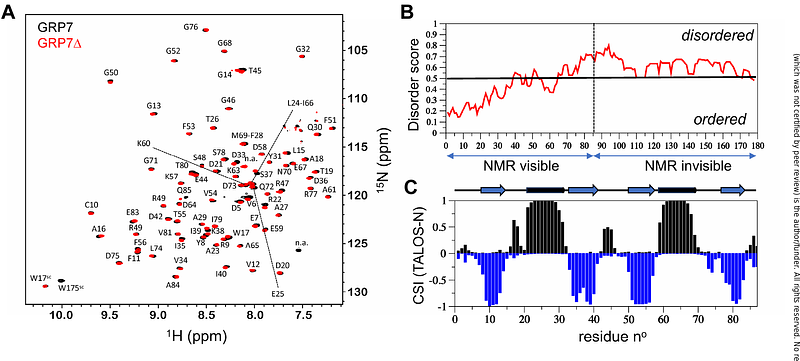
AbstractUranium (U) is a naturally occurring radionuclide, chemotoxic for living organisms. To identify proteins that could be cellular targets for U in plants, we used metalloproteomic approaches combining column chromatographic fractionation analyses, protein identification by high-resolution mass spectrometry shotgun proteomics, and metal quantification by induced coupled plasma mass spectrometry. We identified 57 candidate proteins for uranyl (U(VI)) binding in cultured Arabidopsis thaliana cells. One of these proteins, the Glycine-Rich RNA-binding Protein 7 (GRP7) is a RNA-binding protein involved in various developmental processes and responses to biotic and abiotic stress. Recombinant GRP7 was purified from overproducing bacteria and subjected to further biochemical characterization. First, we showed that GRP7 binds U(VI) with a 1:2 (protein:metal) stoichiometry in vitro. Next, we analyzed its structural properties by solution-state nuclear magnetic resonance spectroscopy. This allowed us to gain insight into the molecular dynamics of the protein-metal interaction and to identify residues involved in U(VI)-binding in the two sites. Finally, we observed that U(VI) binding interferes with nucleic acid binding to the protein RNA-binding domain, suggesting that U(VI) binding to GRP7 contributes to U toxicity in plants.